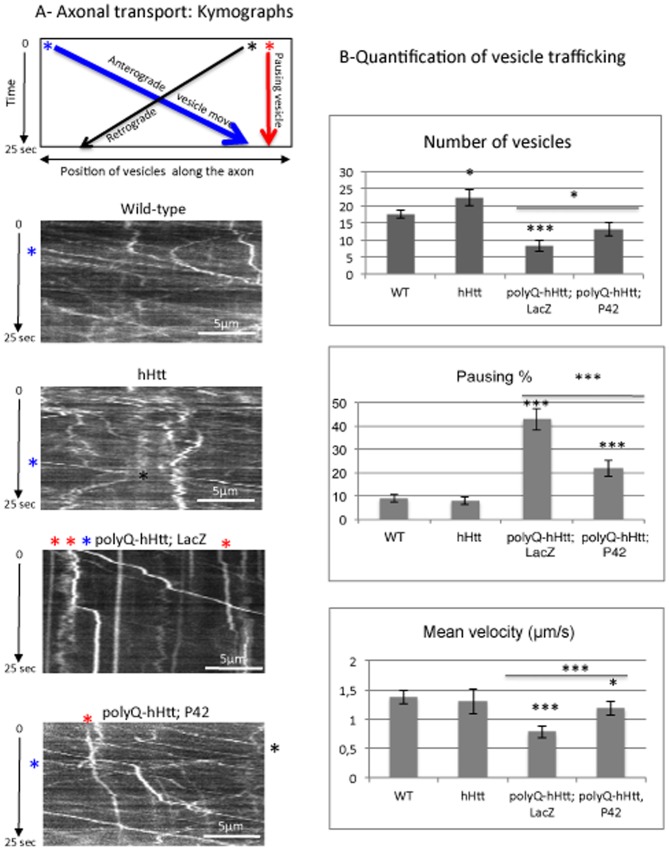
Figure 5. Axonal transport of neuropeptide Y-GFP vesicles.
A- A representative schematic kymograph of vesicular movement along the axons is shown. The data were obtained for vesicles going in anterograde (* in blue) or retrograde (* in black) directions, as well as for vesicles that do not move (* in red). The starting point of vesicles is shown as examples and follow a trajectory according to the color code. Kymographs of vesicular movement are shown for four genotypes: Wild-type, OK6-Gal4/+; UAS-NPY-GFP/UAS-0Q-hHtt548aa (hHtt), OK6-Gal4/UAS-128Q-hHtt548aa; UAS-NPY-GFP/UAS-LacZ (polyQ-hHtt; LacZ), and OK6-Gal4/UAS-128Q-hHtt548aa; UAS-NPY-GFP/UAS-P42 (polyQ-hHtt; P42). Note that only few vesicles are detectable in (polyQ-hHtt; LacZ) larvae and most of them are pausing (* in red), which is not the case in presence of P42. B- Quantification of different parameters (number of vesicles, % of pausing and mean velocity of vesicles) for NPY-GFP vesicular movement was performed in the genetic backgrounds described in A (n = 9-13 larvae). Mean values are compared to wild-type conditions (WT), or to polyQ-hHtt; LacZ. Data were analyzed with the Student’s t-test (*p<0.05, ***p<0.001). As compared to the control (WT), the presence of 128Q-hHtt548aa reduced vesicle number, increased the percentage of pausing, and reduced the mean velocity. Note that the presence of P42 rescued the defects induced by 128Q-hHtt548aa.