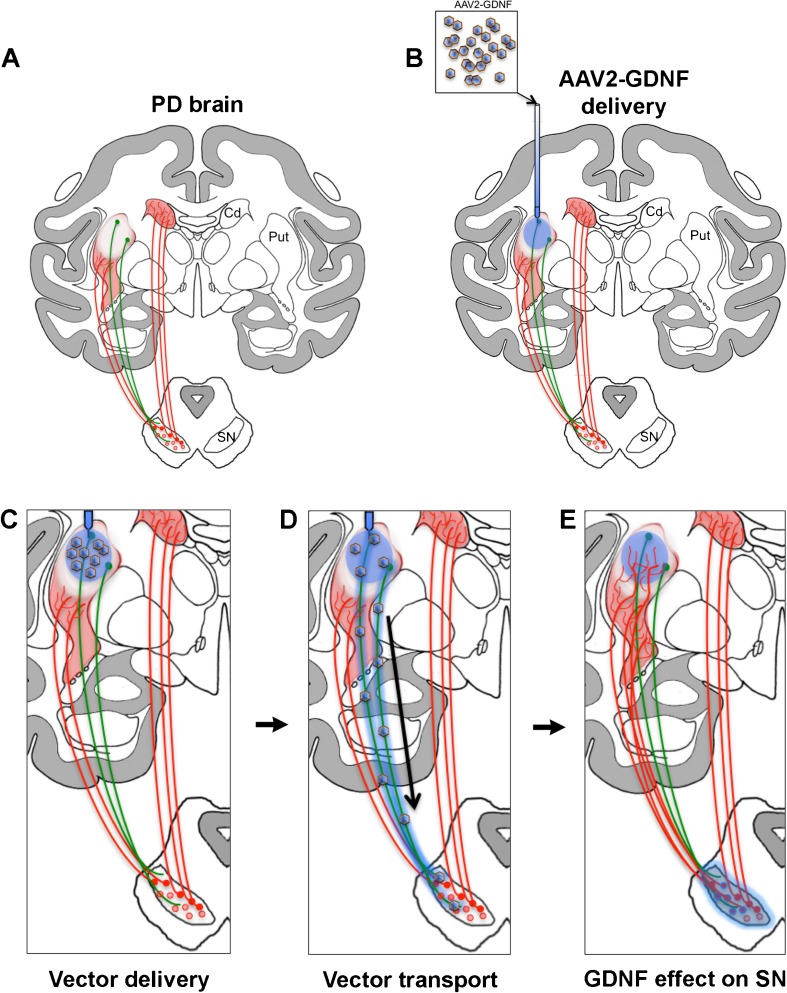
Fig. 1.
Adeno-associated virus 2 (AAV2)–glial cell line-derived neurotrophic factor (GDNF) gene therapy for Parkinson’s disease (PD). (a) Striatal dopamine in Parkinsonian brain (red shadow) is decreased owing to the loss of dopaminergic neurons and fibers from the substantia nigra (SN). Dopamine depletion is greater in the putamen (Put) than in the caudate nucleus (Cd). Gene therapy-based delivery of glial cell line-derived neurotrophic factor (GDNF), a well-known neuroprotective and neurorestorative factor for midbrain dopaminergic neurons (red dots and lines), rescues nigral neurons and, consequently, improves striatal dopaminergic tone in PD experimental models [104]. (b) AAV2 harboring GDNF transgene (blue shadow) is infused into putamen by convection-enhanced delivery to obtain an optimal distribution of the vector. (c) Viral particles transduce striatal medium spiny neurons (green dots and lines). (d) AAV2 is anterogradely transported through axons reaching the SN reticulata. Furthermore, some of the AAV2–GDNF particles are probably transported transynaptically and transduce nigral neurons (red and blue dots in SN). Although AAV2 is an anterograde vector, the dramatic loss of nigrostriatal fibers in the Parkinsonian brain prevents retrograde transport of GDNF protein through this pathway. (e) GDNF trophic effect on nigral neurons promotes neuronal survival and induces nigrostriatal dopaminergic sprouting seen as regrowth of axons in the striatum (note the larger number of dopaminergic fibers in Put) that results in the amelioration of Parkinsonian symptoms