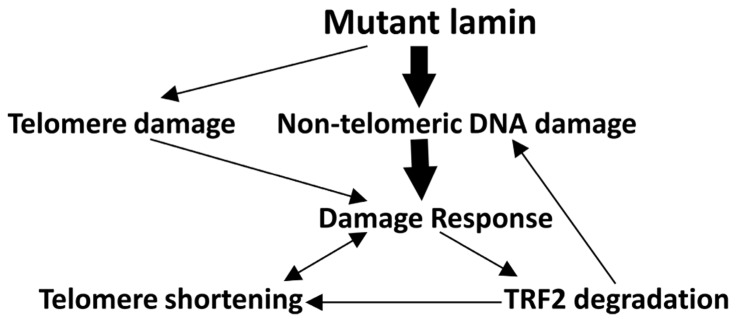
FIGURE 6.
Potential mechanisms ofTRF2 degradation and telomere shortening in LMNA mutant AWS cells. Our data support the hypothesis that in young passage cells, though mutant lamin may initiate some DNA damage to the telomeres, the bulk of the DNA damage accumulates within non-telomeric DNA (bold arrows). The latter may be largely responsible for the initiation of a DNA damage response, followed by TRF2 degradation and destabilization of the telomere–shelterin complex. These alterations in turn cause rapid telomere attrition (Oh et al., 2003) and replicative senescence in these cells. Depletion of TRF2 protein along with insufficient DNA damage repair may also increase the overall levels of genomic DNA damage via a positive feedback loop (Mao et al., 2007). In senescent LMNA mutant cells, all the pathways may be activated.