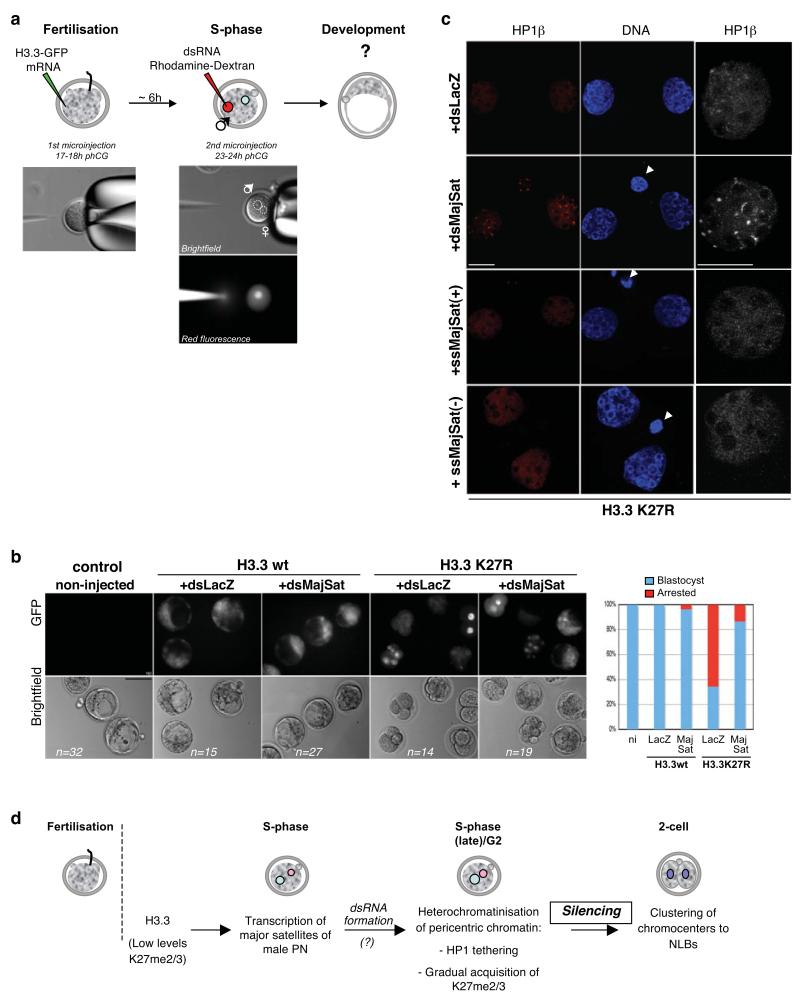
Figure 7. Addition of dsRNA from major satellites in the zygote rescues the developmental phenotype of H3.3 K27R mutants.
a. Experimental design for injection of dsRNA in embryos expressing H3.3 wt or K27R mutant. The first injection of H3.3 wt or H3.3 K27R –GFP constructs was as in Figure 1. Embryos were then cultured for ~6 hours till S-phase, at which time the male pronucleus was microinjected with dsRNA for LacZ (control) or for the major satellite repeat (dsMajSat). To control for pronuclear injection, dsRNA was mixed with rhodamine-coupled dextran.
b. The developmental phenotype elicited by H3.3 K27R is rescued by addition of dsRNA of major satellites. Representative embryos after 3 days of development are shown for each experimental group. The graph on the right depicts the percentage of embryos in each group that reached the blastocyst stage (blue), relative to the control, non-injected (ni) embryos, which was set at 100%. Embryos (%) showing defective development are shown in red. Data are compiled from 4 independent experiments.
c. HP1β relocalises to DAPI-rich regions upon injection of dsRNA for major satellites in H3.3 K27R-expressing embryos. Embryos were injected as in a, fixed at the 2-cell stage and analysed with an HP1β antibody. Shown are representative of 10 embryos analysed per group in two independent experiments. For the ssRNA experiments, results shown are for the sense (+) and the antisense (-) RNA (n=13 and 8). The right panel shows a higher magnification of one of the nuclei shown on the left. Scale bar is 10 μm.
d. Model for heterochromatin establishment at pericentromeric repeats. The presence of H3.3 and low levels/absence of K27 methylation in wild type embryos provide a chromatin environment for transcription of pericentromeric chromatin during the 1st S-phase in the male pronucleus. Both transcription of these domains and gradual accumulation of K27 methylation would subsequently lead to their heterochromatinisation and correct nuclear spatial positioning around NLBs at the 2-cell stage. Reinforcement of histone modifications in the second cell cyle will then allow heterochromatin maintenance.