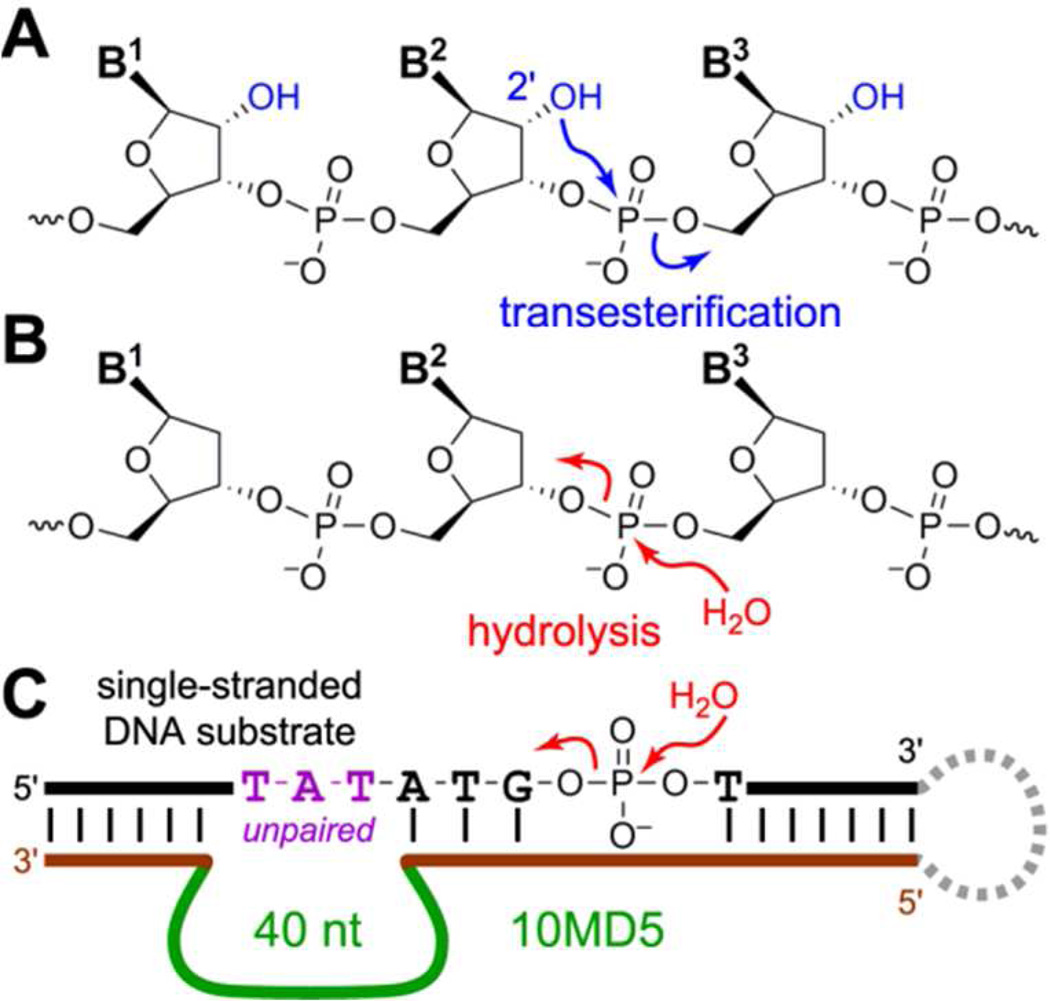
Figure 1.
Oligonucleotide cleavage mechanisms and reaction catalyzed by the 10MD5 deoxyribozyme. (A) RNA cleavage by transesterification at phosphorus. Attack of a ribonucleotide 2′-hydroxyl group at the adjacent phosphodiester linkage leads to 2′,3′-cyclic phosphate and 5′-hydroxyl termini. (B) DNA cleavage by phosphodiester hydrolysis. Attack of a water molecule can form 5′-phosphate + 3′-hydroxyl termini as shown; formation of 3′-phosphate + 5′-hydroxyl is also possible. Competing transesterification cannot occur because no 2′-hydroxyl is present. (C) 10MD5-catalyzed DNA hydrolysis, showing the selection arrangement that enables PAGEshift selection (downward PAGE shift upon substrate cleavage; the dashed loop on the right side enables selection but is dispensable for catalysis). See Figure S1 for details.