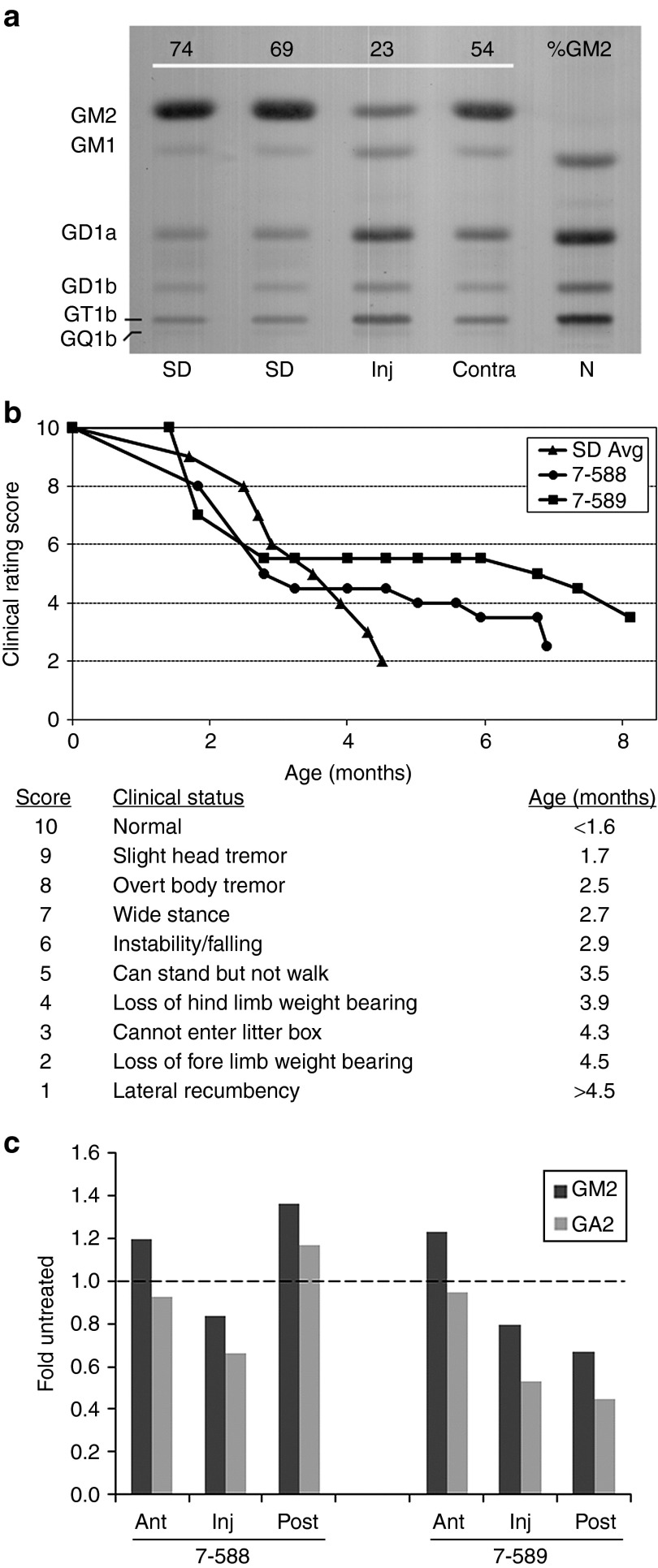
Figure 1.
Therapeutic benefit of human Hex expressed from an AAV1 vector in Sandhoff disease (SD) cats. (a) Cat 7-532 was injected in the right thalamus with a 1:2 ratio of AAV1-CAG-hHEXA/B-Tat-WPRE (A:B = 1 × 1011 g.e.:2 × 1011 g.e., Table 1). Tissue was collected 6 weeks post-injection, and GM2 storage was analyzed by high-performance thin-layer chromatography. Shown are samples from the injected thalamus (Inj), the contralateral thalamus (Contra), and corresponding sections from untreated SD and normal (N) cats. GM2 levels were quantitated densitometrically and expressed as a percentage of total ganglioside on the plate (%GM2). Gangliosides were identified by comparison to known standards and labeled accordingly. The absence of a prominent GM2 band in the normal control lane caused a slight migration shift for GM1 and other bands. (b) Two SD cats treated for long-term follow-up (7-588 and 7-589) had delayed disease progression and increased life span (7.0 and 8.2 months, respectively). Before intracranial injection, cat 7-589 was treated by intravenous injection of AAV8 vectors with a liver-targeted promoter. Disease progression was scored according to a clinical rating scale developed for untreated SD cats, with average age of symptom acquisition compiled from nine separate animals (SD Avg). (c) Total brain lipids were analyzed as in a from the injection site (Inj) and the adjacent anterior (Ant) and posterior (Post) blocks from AAV-treated cats (7-588 and 7-589) at humane endpoint. GM2 (dark bar) and GA2 (light bar) levels were compared with corresponding blocks of an untreated SD cat at its humane endpoint (4.3 months of age). Data for AAV-treated cats is expressed as “fold untreated”, with the untreated level indicated by a dashed line. AAV, adeno-associated virus; g.e., genome equivalents.