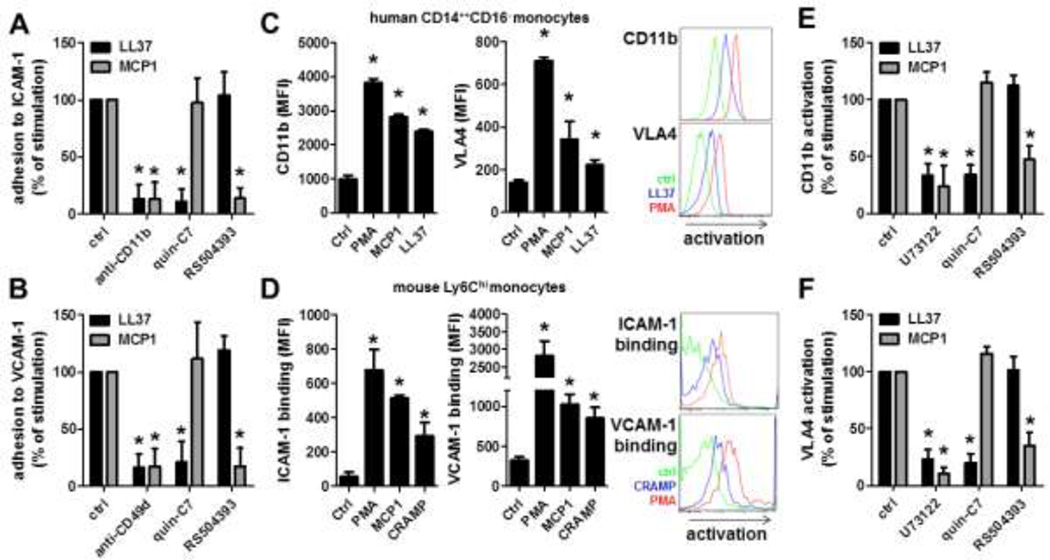
Figure 4. Cathelicidins induce integrin activation.
A/B: LL37 mediates Mac1 (CD11b/CD18) and VLA4 (CD49d/CD29) dependent adhesion. Classical CD14++CD16− monocytes were perfused over P-selectin/ICAM-1 (A) or P-selectin/VCAM-1 (B) coated dishes. The increase in adhesion by coimmobilization of LL37 or MCP1 was set to 100%. Classical CD14++CD16− monocytes were pretreated with anti-CD11b (1µg/ml), anti-CD49d (1µg/ml), or antagonists to FPR2 (quin-C7, 1µM) or to CCR2 (RS504393, 1µM). n=8. * significant difference compared to ctrl. C: Based on the CD16 and CD14 staining properties, human monocyte subsets were identified within peripheral blood mononuclear cells. Moreover, antibodies to activation epitopes of VLA4 (HUTS-21) and CD11b (CBRM1/5) were added. Cells were treated with PMA (50ng/ml), MCP-1 (50ng/ml), or LL37 (1µg/ml) for 15 min and the expression of activated CD11b (left) or VLA4 (right) was assessed on classical CD14++CD16− monocytes. * significant difference from control group. n=6. D: CRAMP activation enhances ICAM-1 and VCAM-1 binding to classical monocytes. Mouse peripheral leukocytes were treated with PMA (50ng/ml), MCP-1 (50ng/ml), or CRAMP (1µg/ml) in the presence of ICAM-1-Fc (left) or VCAM-1-Fc (right) and an anti-Fc antibody. Monocyte subsets were identified by additional antibody staining (CD45, CD11b, CD115, Gr1). * significant difference from control group. n = 5. E/F: Expression of activated CD11b (E) or VLA4 (F) on classical CD14++CD16− monocytes in response to LL37 (1µg/ml) or MCP1 (50ng/ml) was set to 100 %. PBMCs were pretreated with antagonists to PLC (U73122, 100nM), FPR2 (quin-C7, 1µM) or CCR2 (RS504393, 1µM). n=4. * significant difference compared to respective control group.