Abstract
Background
The Acorn CorCap Cardiac Support Device (CSD; Acorn Cardiovascular Inc, St. Paul, MN) is a woven polyester jacket that is placed around the heart and designed to reverse the progressive remodeling associated with dilated cardiomyopathy. However, the effects of the Acorn CSD on myofiber stress and ventricular function remain unknown. We tested the hypothesis that the Acorn CSD reduces end-diastolic (ED) myofiber stress.
Methods
A previously described weakly coupled biventricular finite element (FE) model and circulatory model based on magnetic resonance images of a dog with dilated cardiomyopathy was used. Virtual applications of the CSD alone (Acorn), CSD with rotated fabric fiber orientation (rotated), CSD with 5% prestretch (tight), and CSD wrapped only around the left ventricle (LV; LV-only) were performed, and the effect on myofiber stress at ED and pump function was calculated.
Results
The Acorn CSD has a large effect on ED myofiber stress in the LV free wall, with reductions of 55%, 79%, 92%, and 40% in the Acorn, rotated, tight, and LV-only cases, respectively. However, there is a tradeoff in which the Acorn CSD reduces stroke volume at LV end-diastolic pressure of 8 mm Hg by 23%, 25%, 30%, and 7%, respectively, in the Acorn, rotated, tight, and LV-only cases.
Conclusions
The Acorn CSD significantly reduces ED myofiber stress. However, CSD wrapped only around the LV was the only case with minimal negative effect on pump function. Findings suggest that LV-only CSD and Acorn fabric orientation should be optimized to allow maximal myofiber stress reduction with minimal reduction in pump function.
The Acorn CorCap Cardiac Support Device (CSD; Acorn Cardiovascular Inc, St. Paul, MN) is a bidirectional woven polyester yarn jacket placed over the left (LV) and right ventricles (RV) in patients with dilated cardiomyopathy and heart failure. The underlying hypothesis is that the CSD will reduce strain and stress associated with progressive ventricular dilation. As a consequence, LV remodeling will halt or reverse cardiac remodeling, and systolic LV function will be chronically improved.
A randomized clinical trial of the Acorn CSD has been completed [1, 2]. End-diastolic (ED) and end-systolic (ES) volumes (EDV, ESV) were decreased [2], and there was an improvement in heart failure class [1] that extended to 5 years [3]. These findings occurred when the Acorn CSD was used alone or with mitral valve operations [1, 2]. Although the trial yielded important findings, the exact mechanical effects of the Acorn CSD on ventricular wall stress remain unknown. In addition, prestress on the device and fabric orientation probably have a significant impact on stress and pump function, but have not been evaluated.
Finite element (FE) modeling of the heart and cardiac surgical procedures is becoming more common. However, FE modeling of passive constraint devices, such as the Acorn CSD, is challenging because the device encircles the RV and the LV, and the use of a single LV model is inappropriate. In that regard, we have recently described a weakly coupled biventricular FE and lumped-parameter circulatory system model of heart failure that is appropriate for modeling of a passive constraint device [4].
Measurement of Acorn fabric stiffness is also challenging because the fabric is an open-cell mesh, and continuum mechanics cannot be used for analysis. In a recent study, we embedded the fabric in a silicone matrix and then performed mechanical testing to estimate the fabric material properties [5]. It is worth noting that the Paracor HeartNet Device (Paracor Medical Inc, Sunnyvale, CA) fabric is also an open-cell mesh and significantly anisotropic, similar to the Acorn CSD [6].
We sought to simulate the effects of the Acorn CSD, fabric, and fabric orientation on regional wall stress and pump function. For this purpose, we used a biventricular FE model based on magnetic resonance images of a dog with pacing tachycardia-induced dilated cardiomyopathy. We hypothesize that the Acorn CSD reduces ED myofiber stress.
Material and Methods
Animal Model and Experimental Data
The details of the animal model and data acquisition have been described previously [7]. In brief, a single mongrel dog underwent rapid pacing to induce dilated cardiomyopathy. Magnetic resonance images were taken 4 weeks after pacing was initiated and were contoured using customized FindTags software (Laboratory of Cardiac Energetics, National Institutes of Health, Bethesda, MD) [8].
Biventricular FE Model
The biventricular FE model has been previously described [4]. Briefly, geometric surfaces of the endocardium and epicardium of the LV and RV were created at ED and ES using Rapidform (INUS Technology Inc, Sunnyvale, CA) to compute ventricular volume. Surfaces created at early diastole were used to generate a biventricular FE model (Fig. 1A) using the commercial mesh generator TrueGrid (XYZ Scientific Inc, Livermore, CA). The FE simulations were conducted using LS-DYNA software (LSTC Inc, Livermore, CA).
Fig. 1.
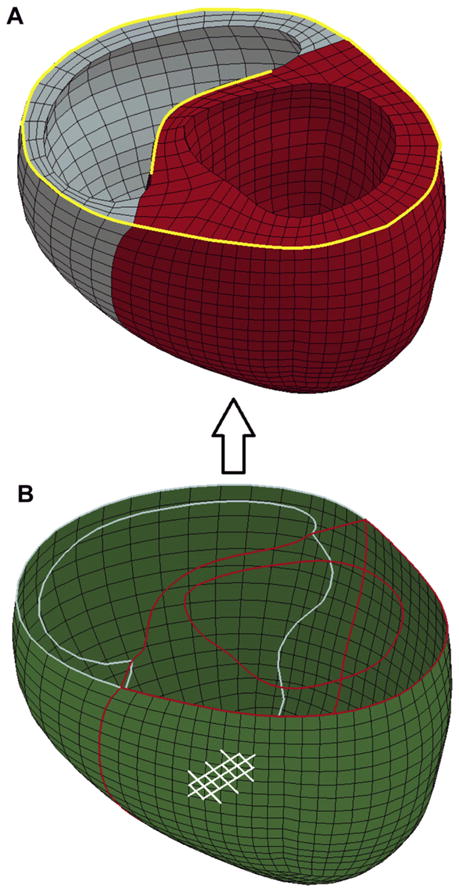
(A) The biventricular finite element model shows the left ventricle in red and the right ventricle in blue. The yellow lines indicate the edges that were fully constrained. (B) The green shell elements represent the Acorn cardiac support device jacket. The contour lines inside of the green shell elements represent the boundaries of the ventricular cavities, and the white lines on the outer shell surface show an example of the fiber orientations.
Boundary and Loading Conditions
The endocardial walls of the LV and RV were loaded with experimentally measured or simulation-based pressures. The boundary conditions of the heart were assigned to constrain the displacement of the basal nodes to deform in the circumferential-radial plane. Additionally, the nodes shown along the yellow curves in Figure 1A were fully constrained.
Material Properties of the Heart
Nearly incompressible, transversely isotropic, hyperelastic constitutive laws for passive [9] and active myocardium [10] were modeled with a user-defined material subroutine [11] in LS-DYNA. The material constant determining the passive compliance of the ventricles (C) [9], and the material constant determining the strength of myofiber contraction (Tmax) [12] were adjusted until the EDV and ESV in the model matched the experimentally observed volumes, as shown previously [8].
Simulation of the Acorn CSD
Virtual applications of the CSD alone (Acorn), CSD with rotated fabric fiber orientation (rotated), CSD with 5% prestretch (tight), and CSD wrapped only around the LV (LV-only) were performed. The fiber orientation of the Acorn case was taken to be approximately 45 degrees and −45 degrees, relative to the circumferential direction, and the rotated case was taken to be approximately 60 degrees and 0 degrees. Both sets of angles are based on previous measurements of the CSD [13].
Acorn Fabric
We recently derived a material property law sufficient to describe the Acorn CSD fabric and measured material constants in silicone embedded fabric samples with a biaxial stretcher [14]. Those findings were implemented into LS-DYNA using the material routine *MAT_091 [8]. Because the material model only captures a single family of fibers, the jacket was modeled using 2 sets of bilinear shell elements to capture both the primary and secondary fibers, using 4,320 elements (Fig. 1B). The nodes of the 2 shell layers were coincident, ensuring that the strain was the same in overlapping elements. Each set of elements represented half the total thickness of the jacket and was assumed to act as a membrane by using a single integration point through the thickness. The total thickness of the shell mesh was assigned to be 0.448 mm, based on measurements of actual CSD samples. The jacket nodes were also coincident with the epicardial surface of the biventricular model, which implies that the jacket does not slip relative to this surface. In the LV-only case, the jacket was assumed to be sutured to the RV insertion.
Acorn Fabric Prestretch
To simulate the effects of tension, due to surgical implantation of the CSD, was added to one of the simulation cases. Specifically, the material properties of the undeformed jacket are specified in the input deck. Then, the FE software applies a 5% prestretch to the jacket at time 0, which decreases the volume of the ventricles and essentially increases the stiffness of the jacket. This is meant to simulate a surgeon “cinching” the jacket before suturing.
Calculation of ED and ES Pressure-Volume Relationships
Families of LV and RV ED pressure-volume relationship (EDPVR) or ES pressure-volume relationship (ESPVR) curves were created at a constant value in the FE model. The resulting data was fit to an exponential equation (Equation 1) and a linear equation (Equation 2) using least squares regression analysis,
| (1) |
| (2) |
where Pd, K, and Vd are the parameters that characterize the diastolic compliance of the ventricle, V0 is the volume intercept, and EES is the slope of the LV elastance.
Circulatory System Modeling
The weakly coupled biventricular FE and lumped-parameter circulatory system model has been previously described [4]. Briefly, to account for the coupling between the biventricular FE model and the vascular system, we implemented an analog circulatory model following the classical model PHYSBE [15] using Simulink (The Mathworks, Natick, MA). The ESPVRs and EDPVRs that were calculated from the FE simulations were used as input for the circulatory system model. This can be thought of as a one-way coupling, where the FE simulations are used to drive the circulatory system model. The details of the circulatory system equations can be found in the Appendix [16].
Calculation of Stroke Volume/EDP (Starling) Relationship
We constructed the Starling relationship by conducting virtual inferior vena cava (IVC) occlusion experiments with the analog circulatory model. We started simulations of the analog circulatory model with 0 initial flows in the system and ran the simulations until it reached a steady state. Next, a virtual IVC occlusion was conducted by inserting a very large resistance component between the outlets of lower body circulation components (trunk and legs) and the inlet of vena cava. The virtual IVC occlusion led to a transient reduction of LV preload. The pressure volume loops after IVC occlusion were recorded, and the Starling curve was constructed by using corresponding pairs of LV EDP and stroke volume data from selected heart beats. Note that our circulatory model parameters were tuned for the experimentally measured hemodynamics data. To obtain the Starling curve in the area beyond the in vivo condition, blood was artificially infused to the circulatory model to increase the ventricular EDP and ESP.
Statistical Analysis
Stress was averaged over all elements of each LV and RV region and presented as the average ± standard deviation in each region. A single FE model based on a single animal was used. The results obtained are not stochastic, and statistical tests were therefore not appropriate; therefore, p values are not reported.
Results
ED Myofiber Stress
The effect of Acorn CSD application on ED myofiber stress is reported in Table 1. The following comparisons are relative to the preoperative case. Briefly, the Acorn CSD has a large effect on ED myofiber stress, with reductions of 55%, 78%, and 47% in the LV free wall, RV free wall, and septum, respectively. If a 5% prestress is applied (tight case), stress reduction is significantly greater, with reductions of 92%, 102%, and 74% in the LV free wall, RV free wall, and septum, respectively. The effect of fabric fiber rotation (rotated case) is interesting because the myofiber stress reductions of 79%, 102%, and 60% in the LV free wall, and RV free wall, and septum, respectively, are significantly greater than in the baseline Acorn case. Also, if the wrap is only applied to the LV (LV-only case), there is a stress reduction in the LV free wall of 40% but increases in stress of 2% and 15% in the RV free wall and septum, respectively.
Table 1.
Comparison of Volume-Averaged Stress (kPa) in the Myocardium and Acorn Fabric at End-Diastolea
| Variable | Pre-op CHF (Mean ± SD) | Acorn (Mean ± SD) | Rotated fibers (Mean ± SD) | Tight Acorn (Mean ± SD) | LV Only (Mean ± SD) |
|---|---|---|---|---|---|
| LV free wall | 2.20 ± 0.77 | 0.99 ± 0.80 | 0.47 ± 0.32 | 0.18 ± 0.94 | 1.33 ± 0.80 |
| RV free wall | 2.81 ± 1.45 | 0.62 ± 0.61 | −0.069 ± 0.29 | −0.057 ± 0.77 | 2.88 ± 1.48 |
| Septum | 1.59 ± 0.93 | 0.85 ± 0.65 | 0.63 ± 0.56 | 0.41 ± 0.52 | 1.83 ± 0.88 |
| CSD fabric | |||||
| LV free wall | 26.1 ± 2.64 | 35.8 ± 3.33 | |||
| RV free wall | 21.3 ± 3.60 | 40.2 ± 6.62 | |||
Note that myocardial stress is in the myofiber direction and fabric stress is in the circumferential direction. Note also that the ventricular pressure was taken from the measured values of the cardiomyopathy case and applied to all of the Acorn cases (left ventricular end-diastolic pressure = 8 mm Hg and right ventricular end-diastolic pressure = 5 mm Hg).
CH = congestive heart failure; CSD = cardiac support device; LV = left ventricle; RV = right ventricle; SD = standard deviation.
Table 1 also shows circumferential stress in the CSD fabric in the Acorn and tight cases. Stress in the fabric is substantially higher than stress in the myocardium. Also, stress in the fabric around the LV increases by about 27%, whereas the stress in the fabric around the RV increases by about 76% in the tight case compared with the Acorn case.
Effect on EDPVR and ESPVR
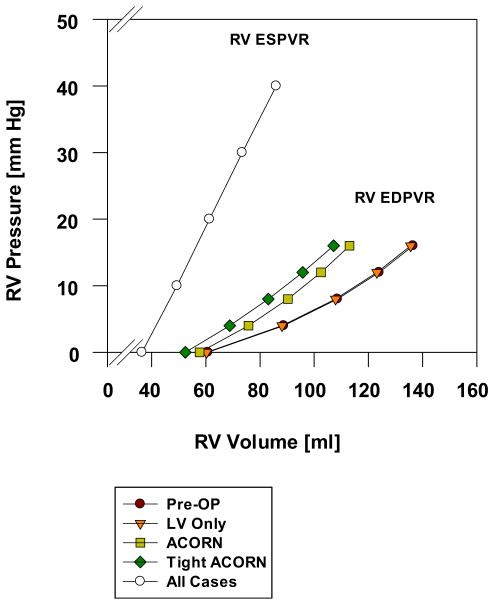
The effect of the Acorn CSD on LV and RV EDPVR is shown in Figures 2 and 3, respectively. In both ventricles, the EDPVR was found to shift to the left. Specifically, the Acorn CSD shifts to the left by 7%, 11%, and 4% in the Acorn, tight, and LV-only cases, respectively. The effect of fabric rotation on LV EDPVR is seen in Figure 2B. There was little effect.
Fig. 2.
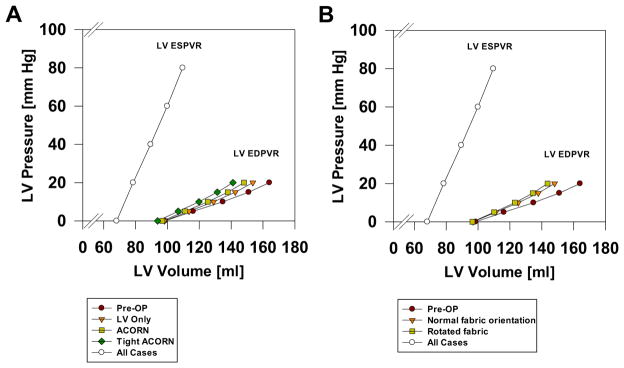
(A) Effect of the Acorn cardiac support device (CSD), an Acorn with 5% (tight Acorn,) and an Acorn applied only to the left ventricle (LV Only) on end-systolic (ESPVR) end-diastolic pressure-volume relationship (EDPVR). The tight Acorn had the greatest effect, whereas the LV-only case has the least effect on LV EDPVR. The ESPVR was not changed by Acorn application. (B) Effect of the Acorn CSD fabric orientation on LV EDPVR. Fabric orientation had minimal effect.
Fig. 3.
Effect of the Acorn cardiac support device, an Acorn with 5% prestretch (tight Acorn) and an Acorn applied only to the left ventricle (LV only) on right ventricular (RV) end-diastolic (EDPVR) and end-systolic pressure-volume relationship (ESPVR). The tight Acorn had the greatest effect, whereas the LV- only case had the least effect on RV EDPVR. The ESPVR was not changed by Acorn application.
The Acorn CSD shifts the measured at RVEDP = 8 and LVEDP = 8 mm Hg to the left by 16% and 23% in the Acorn and tight cases, respectively. There was little effect on volume in the LV-only case (Fig. 3).
CSD application did not impact the ESPVR of either ventricle.
Effect on Pump Function (Starling’s Relationship)
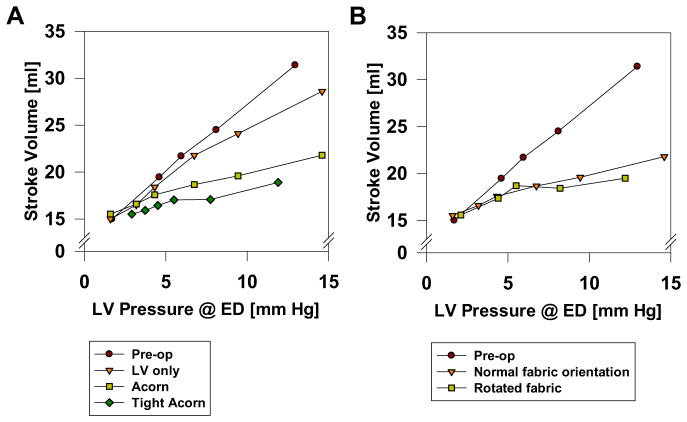
The effect of the Acorn CSD on pump function is shown in Figure 4A. The combined effect of a leftward shift of EDPVR and intact ESPVR leads to the downshift of Starling curve in all cases. Specifically, the Acorn CSD reduces stroke volume at LVEDP = 8 mm Hg by 23%, 30%, and 7% in the Acorn, tight, and LV-only cases, respectively. The effect of fabric rotation on LV pump function is seen in Figure 4B. There was little effect.
Fig. 4.
(A) Effect of the Acorn cardiac support device (CSD), an Acorn with 5% (tight Acorn) and an Acorn applied only to the left ventricle (LV only) on Starling’s law. Tight Acorn had the greatest effect, whereas the LV-only case has the least effect on LV end-diastolic (ED) pressure-volume relationship. (B) Effect of the Acorn CSD fabric orientation on Starling’s law. Fabric orientation had minimal effect.
Comment
The principal finding of the current study is that the Acorn CSD significantly reduces ED myofiber stress. However, CSD wrapped only around the LV was the only case with minimal negative effect on pump function.
Effect on Fiber Stress at ED
Our FE-based simulation shows that application of the Acorn CSD causes a large reduction in ED myofiber stress. The high stress in the Acorn CSD fabric that occurs in our simulations is surprising even given that the fabric is much thinner than the LV and RV walls. This shows that the Acorn CSD reduces myofiber stress at ED by reducing LV volume at ED but also by acting as a structural element that shares the load with the myocardium.
Effect on Diastolic Compliance
Acute reduction in diastolic compliance without a corresponding increase in systolic elastance leads to a reduction in pump function. Reduction in diastolic compliance without a change in systolic elastance was also seen in our previous FE simulation of adjustable passive constraint [13] and is similar to the effect of surgical ventricular remodeling on diastolic compliance and pump function [17, 18]. These findings suggest that acute reduction in ventricular compliance with subsequent reduction in pump function is a characteristic of not only all passive constraint procedures but also of surgical ventricular in general.
Acorn Fabric Prestretch
There are two primary factors that together determine the effect of a fabric-type passive constraint device on diastolic function. The first is the compliance of the fabric per se and the second is the amount of fabric at the time of implantation. If significant fiber occurs at the time of implantation, diastolic compliance of each ventricle may be reduced, with detrimental effects. At the current time, the amount of pretension placed on the Acorn CSD fabric at the time of implantation may reduce by up to 10% [19]. Our model assumes that the slack length of the Acorn fabric is at early diastolic filling and includes fabric prestretch of 5%. However, the optimal amount of prestretch is unknown.
Fabric Orientation
In addition to fabric stiffness, the orientation of the primary and secondary fabric fiber groups, relative to the fabric and to the heart, is important. For instance, orientation of the Acorn CSD so that it is stiffest in the circumferential direction may counteract spherical remodeling [20]. As noted above, the effect of fabric fiber rotation in our study is interesting because the myofiber stress reductions are significantly greater without a different effect on compliance and pump function than in the baseline Acorn case. In the rotated case, one of the primary fabric fiber angles is in the circumferential direction. This case showed the best balance of stress reduction and decreased stroke volume. Our model allows the future determination of optimal and CSD fabric orientation.
LV-Only Acorn CSD Wrap
Our simulation showed that the Acorn CSD applied to the LV only causes a modest reduction in myofiber stress in the LV free wall but minimal stress reduction in the septum and RV. Also, the Acorn CSD applied to the LV only has the smallest effect on LV diastolic compliance and minimal effect on RV compliance. The Acorn CSD applied to the LV only has the smallest negative effect on pump function.
Failure To Affect ES Elastance
Our FE simulations showed that application of the Acorn CSD did not alter the ESPVR. Although preclinical and clinical studies have both described a decrease in ESV after Acorn CSD application [2, 21], we conclude that any improvement in systolic function is not caused by the acute mechanical effect of the Acorn CSD.
Our FE simulation results reveal that the Acorn CSD significantly reduces the ED fiber stress at the endocardium and middle-wall. Changes on preload myofiber stress have been shown to alter myofiber remodeling. The reduction of ED fiber stress likely initiates a positive remodeling process of the ventricles that leads to improved systolic function.
Study Limitations
First, only one animal data set was used to develop the multiscale FE circulatory system model. Second, there is no good large animal model of dilated cardiomyopathy. Hence, it was necessary to use flawed models, such as tachycardia-induced cardiomyopathy, where the dysfunction resolves when the pacing is discontinued.
Conclusion and Future Directions
The Acorn CSD greatly reduces ED wall stress but depresses pump function at the time of implant. Our study suggests that wrap tightness may be optimized when the Acorn CSD is implanted to reduce wall stress without impairing ventricular function. Also the results of this study show that a CSD that only wraps around the LV will yield the best result. Our current study lays the groundwork for studies of the Acorn CSD and Paracor HeartNet Device in which the fabric angles and orientation of the fabric relative to the RV and LV are optimized.
Acknowledgments
This work was supported by National Institutes of Health Grants 5R01 HL077921 to J.M.G., primary investigator, and 5R01 HL063348 to M.B.R., primary investigator.
Appendix
Circulatory Model
The original PHYSBE model approximates the left (LV) and right ventricles (RV) as two chambers with time-varying compliance. PHYSBE’s time-varying compliance model does not reproduce the physiologic end-systolic pressure-volume relationship (ESPVR). To overcome this, we used the approach described by Ursino and colleagues [16] to reproduce this physiologic ESPVR. The RV and LV were modeled in the same fashion, with the only difference being a set of parameters governing the end-systole and end-diastole ventricle pressure-volume relationship (EDPVR). In what follows, we use the LV to describe our ventricle model, although it is also applicable to the RV. The LV pressure within a cardiac cycle is governed by
| (A2) |
where f(t) is a squared half-sine wave that controls the contraction and relaxation of the ventricle [16]. Pact,LV is the maximum LV pressure generated by active contraction of myofibers and is determined by Equation 2 from the finite element model. Ppas,LV is the passive component of LV described as in Equation 1. Note that the LV EDPVR depends on the magnitude of RV EDP and vice versa. Such an interaction between the 2 ventricles was modeled by linear interpolation using the family of LV EDPVRs generated from the finite element model.
References
- 1.Mann DL, Acker MA, Jessup M, Sabbah HN, Starling RC, Kubo SH. Clinical evaluation of the CorCap cardiac support device in patients with dilated cardiomyopathy. Ann Thorac Surg. 2007;84:1226–35. doi: 10.1016/j.athoracsur.2007.03.095. [DOI] [PubMed] [Google Scholar]
- 2.Starling RC, Jessup M, Oh JK, et al. Sustained benefits of the CorCap cardiac support device on left ventricular remodeling: three year follow-up results from the Acorn clinical trial. Ann Thorac Surg. 2007;84:1236–42. doi: 10.1016/j.athoracsur.2007.03.096. [DOI] [PubMed] [Google Scholar]
- 3.Mann DL, Kubo SH, Sabbah HN, et al. Beneficial effects of the CorCap cardiac support device: five-year results from the Acorn trial. J Thorac Cardiovasc Surg. 2012;143:1036–42. doi: 10.1016/j.jtcvs.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wenk JF, Ge L, Zhang Z, et al. A coupled biventricular finite element and lumped-parameter circulatory system model of heart failure. Comput Methods Biomech Biomed Engin. 2012 doi: 10.1080/10255842.2011.641121. [E-pub ahead of print] http://dx.doi.org/10.1080/10255842.2011.641121. [DOI] [PMC free article] [PubMed]
- 5.Chitsaz S, Wenk JF, Ge L, et al. Material properties of Cor-Cap passive cardiac support device. Ann Thorac Surg. 2013;95:148–54. doi: 10.1016/j.athoracsur.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magovern JA. Experimental and clinical studies with the Paracor cardiac restraint device. Semin Thorac Cardiovasc Surg. 2005;17:364–8. doi: 10.1053/j.semtcvs.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Potter DD, Araoz PA, Ng LL, et al. Cardiotropin-1 and myocardial strain change heterogeneously in cardiomyopathy. J Surg Res. 2007;141:277–83. doi: 10.1016/j.jss.2006.12.539. [DOI] [PubMed] [Google Scholar]
- 8.Halquist JO. LS-DYNA Theory Manual. 2006. [Google Scholar]
- 9.Guccione JM, McCulloch AD, Waldman LK. Passive material properties of intact ventricular myocardium determined from a cylindrical model. J Biomech Eng. 1991;113:42–55. doi: 10.1115/1.2894084. [DOI] [PubMed] [Google Scholar]
- 10.Guccione JM, Waldman LK, McCulloch AD. Mechanics of active contraction in cardiac muscle: part II—cylindrical models of the systolic left ventricle. J Biomech Eng. 1993;115:82–90. doi: 10.1115/1.2895474. [DOI] [PubMed] [Google Scholar]
- 11.Sun K, Stander N, Jhun CS, et al. A computationally efficient formal optimization of regional myocardial contractility in a sheep with left ventricular aneurysm. J Biomech Eng. 2009;131:111001. doi: 10.1115/1.3148464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guccione JM, Costa KD, McCulloch AD. Finite element stress analysis of left ventricular mechanics in the beating dog heart. J Biomech. 1995;28:1167–77. doi: 10.1016/0021-9290(94)00174-3. [DOI] [PubMed] [Google Scholar]
- 13.Jhun CS, Wenk JF, Zhang Z, et al. Effect of adjustable passive constraint on the failing left ventricle: a finite-element model study. Ann Thorac Surg. 2010;89:132–7. doi: 10.1016/j.athoracsur.2009.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chitsaz S, Wenk J, Ge L, et al. Material properties of CorCap passivecardiac support device. Ann Thorac Surg. 2013;95:148–54. doi: 10.1016/j.athoracsur.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLeod J. PHYSBE.. a physiological simulation benchmark experiment. Simulation. 1966;7:324–9. [Google Scholar]
- 16.Ursino M. Interaction between carotid baroregulation and the pulsating heart: a mathematical model. Am J Physiol Heart Circ Physiol. 1998;275:1733–47. doi: 10.1152/ajpheart.1998.275.5.H1733. [DOI] [PubMed] [Google Scholar]
- 17.Burkhoff D, Wechsler AS. Surgical ventricular remodeling: a balancing act on systolic and diastolic properties. J Thorac Cardiovasc Surg. 2006;132:459–63. doi: 10.1016/j.jtcvs.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 18.Zhang P, Guccione JM, Nicholas SI, et al. Left ventricular volume and function after endoventricular patch plasty for dyskinetic anteroapical left ventricular aneurysm in sheep. J Thorac Cardiovasc Surg. 2005;130:1032–8. doi: 10.1016/j.jtcvs.2005.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konertz WF, Shapland JE, Hotz H, et al. Passive containment and reverse remodeling by a novel textile cardiac support device. Circulation. 2001;104(12 suppl 1):I270–5. doi: 10.1161/hc37t1.094525. [DOI] [PubMed] [Google Scholar]
- 20.Lembcke A, Dushe S, Dohmen PM, et al. Early and late effects of passive epicardial constraint on left ventricular geometry: ellipsoidal re-shaping confirmed by electron-beam computed tomography. J Heart Lung Transplant. 2006;25:90–8. doi: 10.1016/j.healun.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Saavedra WF, Tunin RS, Paolocci N, et al. Reverse remodeling and enhanced adrenergic reserve from passive external support in experimental dilated heart failure. J Am Coll Cardiol. 2002;39:2069–76. doi: 10.1016/s0735-1097(02)01890-9. [DOI] [PubMed] [Google Scholar]