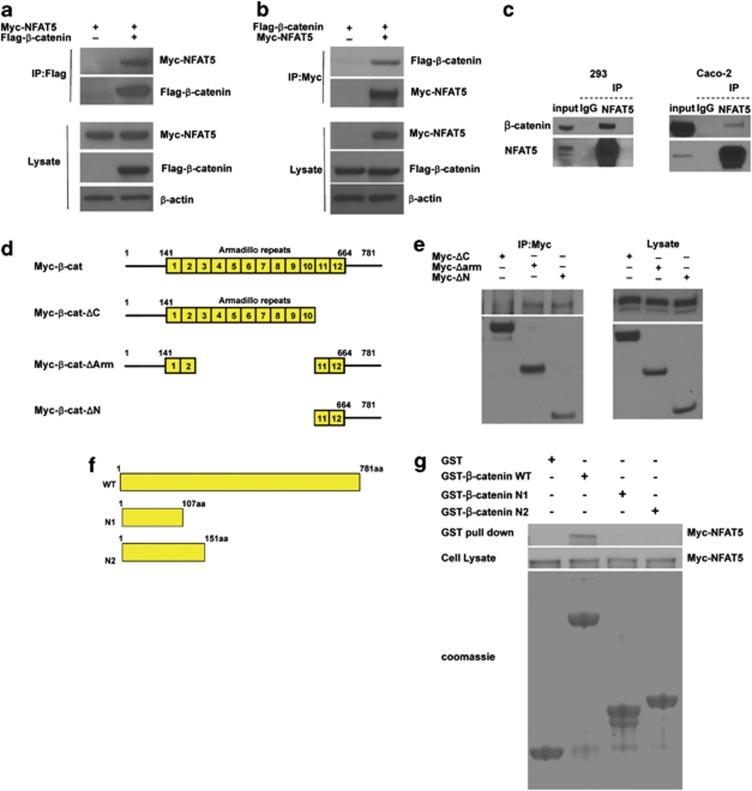
Figure 2.
NFAT5 interacts with β-catenin. (a and b) NFAT5 interacts with β-catenin. HEK293 cells were co-transfected with Myc-tagged NFAT5 and empty vector or Flag-tagged β-catenin, and incubated for 48 h. After anti-Flag (a) or anti-Myc (b) immunoprecipitation, the presence of NFAT5 and β-catenin were analyzed using anti-Myc and anti-Flag antibodies, respectively. Western blot was performed on cell lysate as a control. (c) Interaction between endogenous NFAT5 and β-catenin. Endogenous NFAT5 was immunoprecipitated from HEK293 or Caco-2 cells, and Western blot was performed on the eluate. IgG was used as a negative control. (d) Schematic diagram of β-catenin deletion constructs. The yellow boxes are armadillo repeats. (e) C-terminal-transactivating domains of β-catenin bind NFAT5. HEK293 cells were co-transfected with Myc-tagged β-catenin deletion mutants. Myc-tagged β-catenin mutants were immunoprecipitated using an anti-Myc antibody. Expression of NFAT5 was analyzed by western blot with an anti-NFAT5 antibody. The expression of these β-catenin mutants was analyzed by western blot with an anti-Myc antibody. Western blot was performed on cell lysate as a control. (f) Schematic diagram of the wild-type and mutant derivatives of GST- tagged β-catenin. (g) NFAT5 directly interacts with β-catenin. HEK293 cells were transfected with Myc-NFAT5 and after 48 h incubation, lysate was collected and incubated with the GST protein or GST-tagged β-catenin wide type (WT) or mutant proteins. After several washings, bound proteins were eluted and western blot assay was performed using an anti-Myc antibody