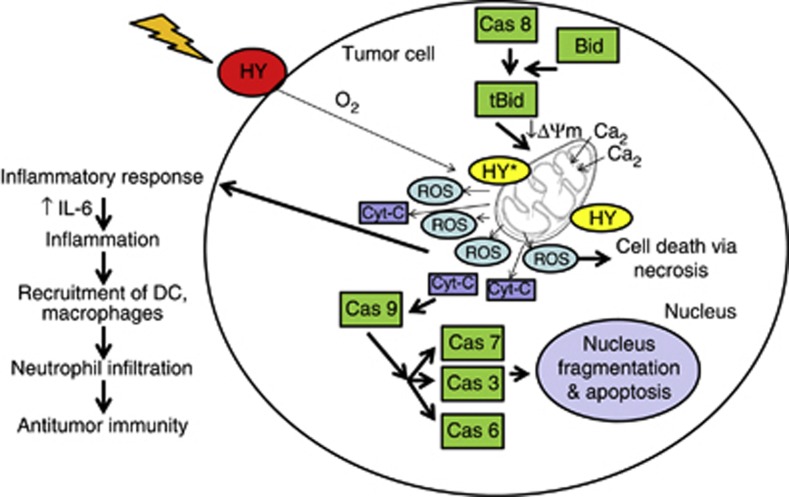
Figure 8.
Proposed model of HY-PDT-induced cell death. We propose a mechanism of tumor cell killing by HY-PDT. The HY, lipophilic molecule, accumulates specifically in the tumor cell membrane42 and enters into the cells via endocytosis, pinocytosis or passive diffusion. The HY binds to mitochondria and become activated following irradiation with visible light. These activated HY react with O2 to produce cytotoxic reactive oxygen species (ROS), responsible for direct tumor cell killing. The HY-PDT results in rapid initiation of an inflammatory response eventually recruiting mediators of inflammation and stimulating tumor cells to release secondary inflammatory mediators for instance, IL-6, TNF-α and IFN-γ in treated cells. The treatment could also promote the accumulation of DC, macrophages and neutrophils leading to tumor destruction.43 Overall, HY-PDT is considered vital for the activation of antitumor immunity. On the other hand, induction of ROS also inclines tumor cell death via the mitochondrial apoptotic pathway. First, release of Ca2+ from the endoplasmic reticulum43, 44 and mitochondria, which has a major role in apoptosis followed by loss of mitochondrial membrane integrity and release of cytochrome c. The Bid cleavage coincided with translocation of tBid from cytoplasm to mitochondria and remarkably activates initiator caspases (caspase-8 or caspase-9) leads to the activation of effector caspases (caspase-3, -6 and -7). The prolonged oxidative stress resulting digestion of nucleosomal DNA into oligonucleosomes and visualize as DNA laddering at the late stage of apoptosis. Under certain circumstances, PDT-treated tumor cells could also undergo necrosis