Figure 4.
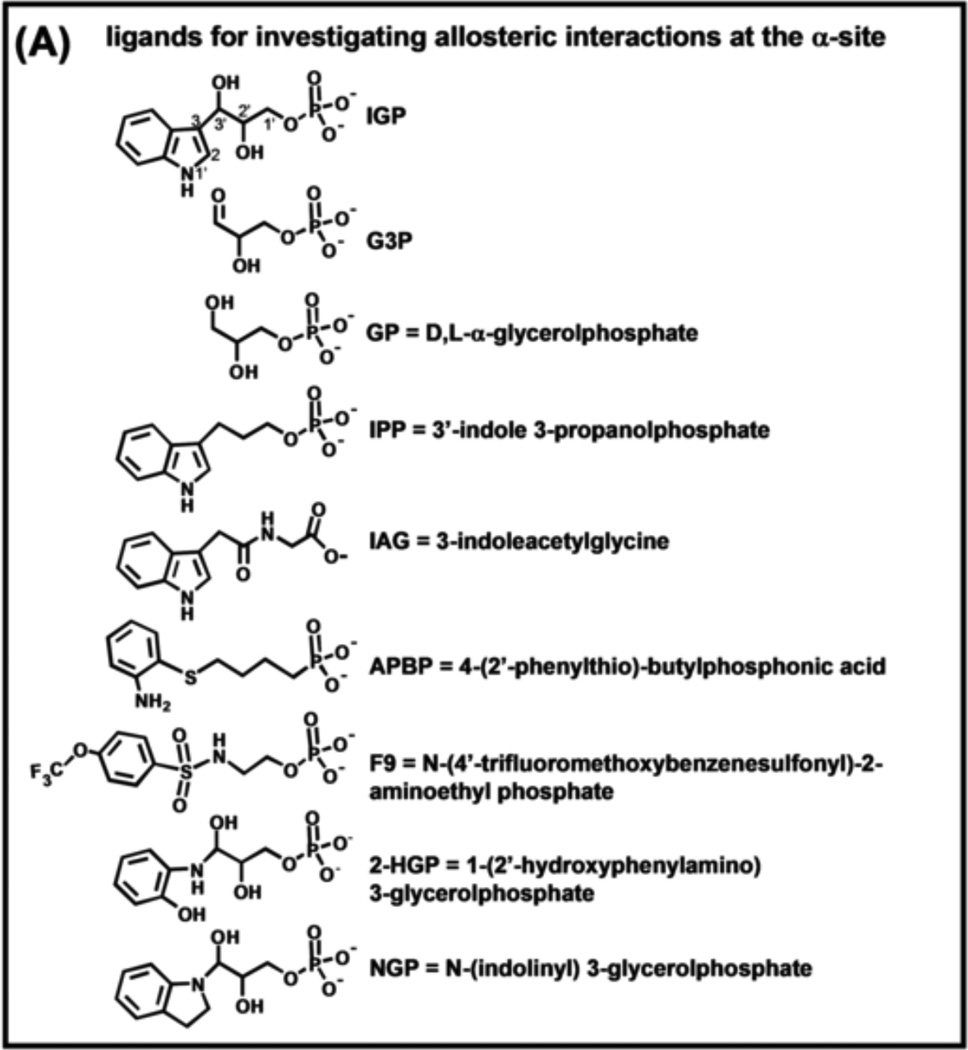
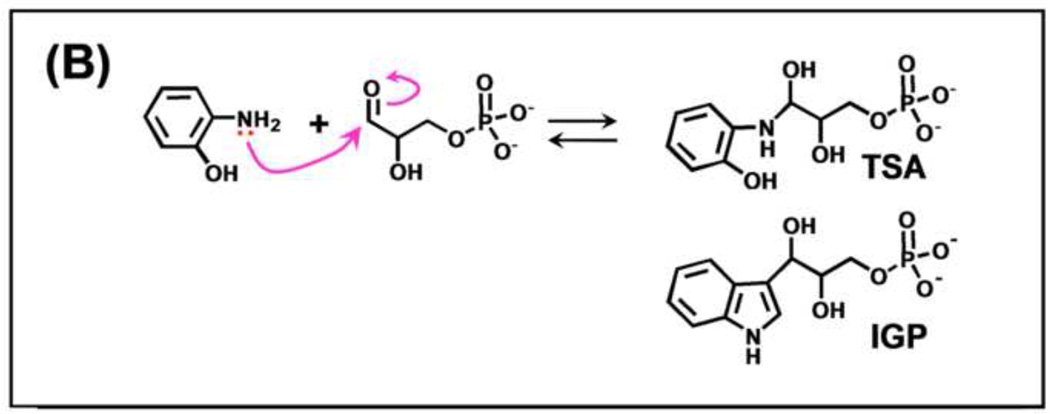
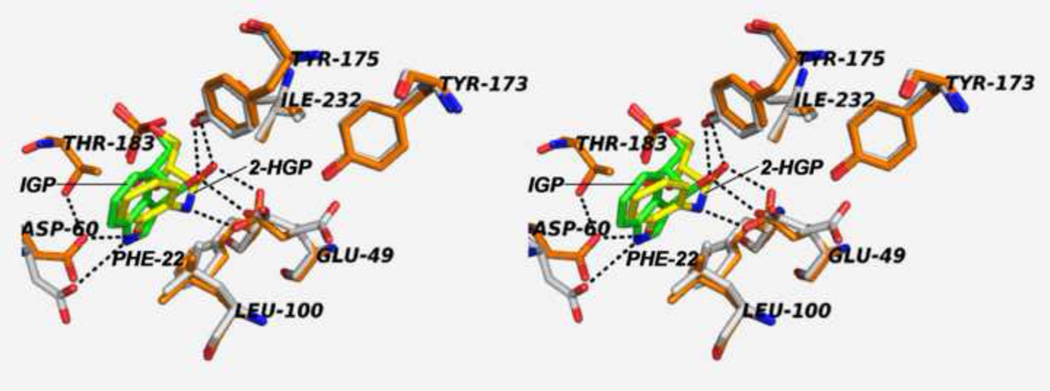
(A) Organic structures and names of some useful α-site ligands. APBP [52], 2-HGP [55] and NGP [27, 47] have been proposed to be transition state analogues for the α-reaction. (B) Reaction of 2-aminophenol with G3P to give the proposed transition state analogue, 2-HGP at the α-site [54]. (C) Stereo structural diagram comparing important features of the α-sites of the complex of 2-HGP (PDB ID: 1TJP) with the complex of IGP (PDB ID: 1QOQ). Color scheme: protein residues of the 2-HGP complex are shown with CPK coloring; 2-HGP, C yellow, N, O, and P in CPK colors. The protein residues of the IGP complex are shown with C orange, N, and O in CPK colors; IGP, C green, N, O, and P in CPK colors. Proposed hydrogen-bonding interactions are show as black dashed lines. Notice that the αD49 carboxylate takes up two orientations in the IGP complex, one with a hydrogen bond between the αD49 carboxylate and the 3-hydroxyl of IGP, the other with the carboxylate rotated by approximately 100° away from IGP.