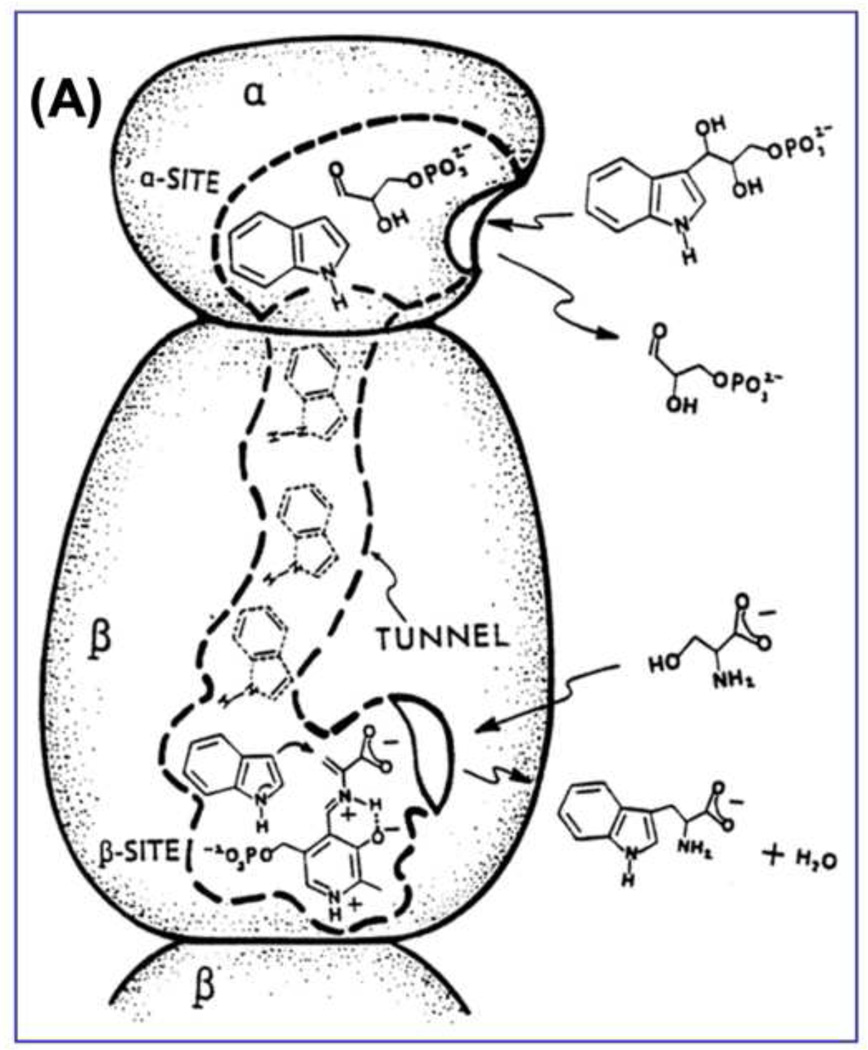
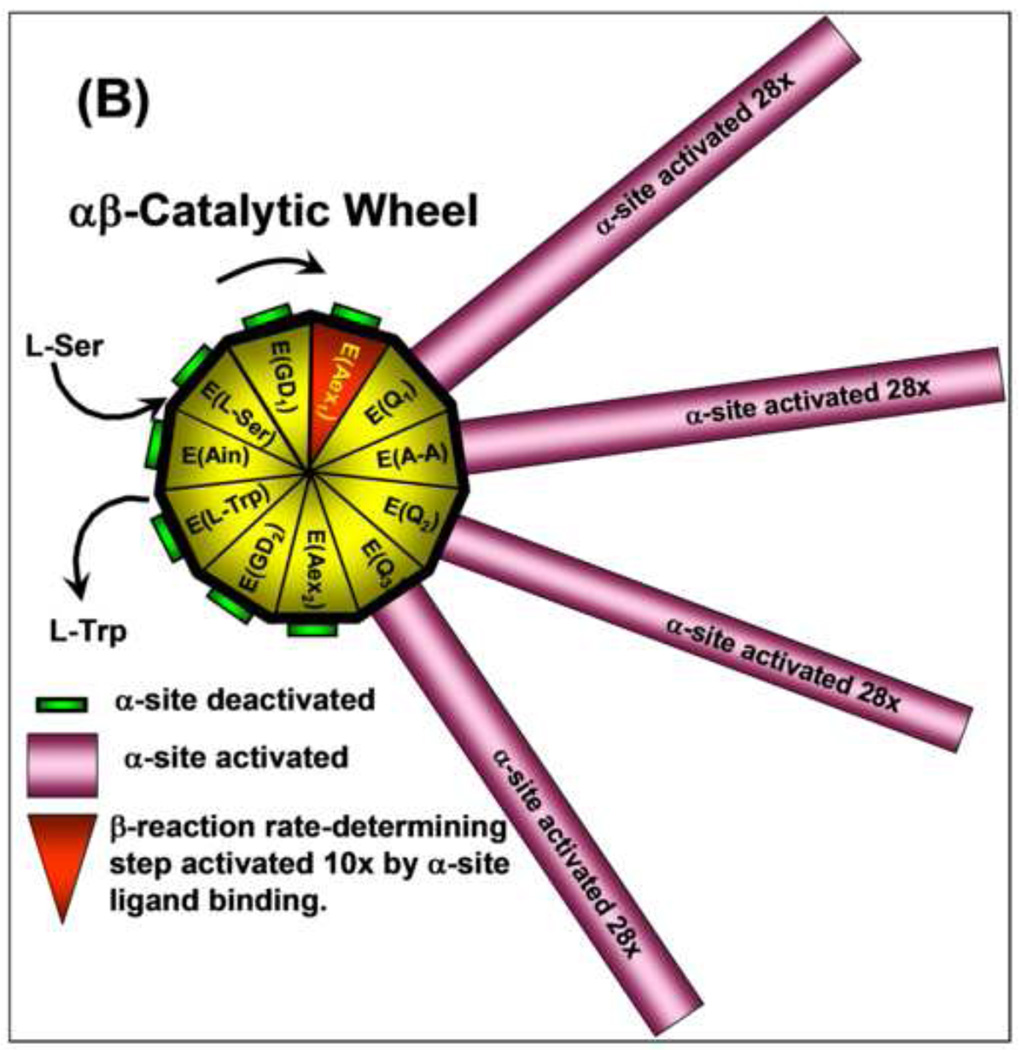
Figure 8.
(A) Cartoon for the αβ-dimeric unit of tryptophan synthase depicting the binding and dissociation of ligands to the α- and β-sites and the transfer of intermediate indole from the α-site to the β-site (redrawn from [62]).
(B) Catalytic wheel summarizing the interplay of ligand binding and reaction with the allosteric signaling between the α- and β-sites during the catalytic cycles of the α- and β-reactions. The gold and red triangular segments of the wheel hub represent the different known chemical states of the β-catalytic cycle (see Figure 1). The green or magenta bars extending out from each triangular segment have lengths proportional to the relative activity of the α-site for each chemical state of the β-site. As the E(Ain) species is converted by reaction with L-Ser to E(GD1) and E(Aex1) the α-site remains in a catalytically inactive state. When E(Aex1) is converted to E(A–A), via E(Q1), the α-site is activated at least 28-fold [61], and remains activated until E(Q3) is converted to E(Aex2) when the α-site is switched off again [63]. As the β-reaction continues from E(Aex2) to the complex of E(Ain) with L-Trp, the α-site remains deactivated. The triangular segment designated as E(Aex1) is colored red to signify activation of the conversion of this intermediate to E(A–A) by IGP binding to the α-site [27]. Modulation of the switching of the α- and β-sites between open states of low activity and closed states of high activity by ligand binding and reaction provides a mechanism for both synchronizing the α- and β-reactions and preventing the escape of indole from the confines of the α- and β-sites and the interconnecting tunnel. This conformational switching makes possible the efficient channeling of indole during the αβ-catalytic cycle of tryptophan synthase. Figure redrawn from [27].