Abstract
Introduction
Despite the increasing incidence of melanoma little is known about patients' emotional distress associated with this disease. Supplemented by the problem list (PL), the distress thermometer (DT) is a recommended screening instrument to measure psychosocial distress in cancer patients. Our objective was to explore the acceptance and the feasibility of the DT and PL as a concise screening tool in an ambulatory setting for routine care and to elucidate determinants of distress in melanoma patients with regard to sociodemographic and clinical variables.
Methods
Consecutive melanoma outpatients were asked to complete the DT with the PL prior to their scheduled consultation. Demographic and clinical data were obtained from the patients' charts. Clinical data included melanoma stage, time since diagnosis, previous treatment, current treatment, and other cancer disease.
Results
Out of 734 patients recruited into the study, 520 patients (71%) completed both the DT and the PL. Forty-seven percent met the ≥5 cut-off score for distress. Younger and employed patients reported higher distress than older and retired patients. A cut-off score of ≥5 was closely associated with self-reported emotional sources of distress, with practical problems, especially at work, family problems (dealing with the partner), and physical problems like pain, appearance, getting around, and nausea. Apart from higher distress under current systemic treatment, no associations were found between distress and clinical data.
Conclusion
The DT together with the PL seems to be an economically reasonable screening tool to measure psychosocial distress in melanoma patients. In particular, younger melanoma patients who are currently employed are prone to experience distress at some point after diagnosis, but there appears to be almost no association between clinical data and the extent of distress. To characterize the impact of distress on disease outcome and quality of life in melanoma patients, further research is needed.
Introduction
The National Comprehensive Cancer Network (NCCN) defines distress as a “multifactorial, unpleasant emotional experience of a psychological (cognitive, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms and its treatment” [1], [2]. Malignant melanoma is the sixth most frequent form of cancer in the USA, which has continuously increased reaching up to 22.2 per 100,000 population in 2008 [3] and usually appears during middle adulthood. Particularly common in fair-skinned populations [3], its occurrence and prognosis are strongly related to behavioral factors, especially sun exposure patterns and the use of indoor tanning booths. Despite a deepening understanding of melanoma tumor biology and promising advances in treatment, surprisingly little is known about the psychological impact melanoma has on patients' lives. While the vast majority of cases are detected at an early stage and therefore treated effectively, recurrence remains a significant risk over many years. Thus, patients suffering from malignant melanoma have to grapple with an ongoing threat. Regular aftercare is therefore recommended for 10 years under the current German melanoma S3 guideline [4], including psycho-oncological treatment.
Yet, distress in malignant melanoma has remained understudied. Estimates of distress vary widely in patients with malignant melanoma. In a systematic review [5], the proportion of participants scoring in the clinical range for anxiety based on the Hospital Anxiety and Depression Scale (HADS) ranged from 18 to 44%, for depression symptoms the range was 6 to 28%. Risk factors for heightened distress were female sex, younger age, the absence of a spouse or partner, and lower levels of education. Surprisingly, stage of disease was unrelated, but physical deterioration and visibility of body site were associated with altered body image and fear of distress [6]. Further associations of distress were found with lack of social support, negative cognitive appraisal and an avoidant coping style [7].
There is evidence that psychological distress is associated with decreased adherence to treatment regimes, lower quality of life, reduced enrollment in follow-up programs, delay in seeking medical advice, increased recurrence rates and mortality, and increased medical costs [8]–[18]. Psychological distress, however, is often overlooked by physicians for many reasons. Patients are often reluctant to ask for help because they fear being stigmatized for having a psychological problem. They do not want to distract physicians from curing their cancer by mentioning psychosocial needs or fear being seen as overly demanding or difficult [13], [19]. Symptoms associated with distress, anxiety, or depression like loss of appetite, fatigue or insomnia might be confounded by symptoms of malignancy or treatment side effects, and the medical staff are not always trained or skilled in perceiving and discussing emotional problems [20]–[22]. As the treatment of melanoma has increasingly been shifted to ambulatory care settings, physician consultations are shortened, thus limiting time to explore emotional well-being. Therefore, the development of screening strategies to improve the detection and management of psychological distress has become even more important.
Recommendations for melanoma surveillance in German skin cancer centers include screening, evaluation, and treatment of distress of melanoma patients [23]. Distress has been studied by validated standardized screening tools, such as the HADS or the Brief Symptom Inventory (BSI) [24]–[27]. Despite their relative brevity, however, these multi-item measures still require more time than is available in busy outpatient skin cancer centers. To improve and implement psycho-oncological care in routine melanoma care programs, the development of brief screening tools to detect psychological distress and the identification of risk factors for distress are urgent needs. Recognizing the need for economical means to screen rapidly for distress in cancer patients, Roth and colleagues developed the single-itemed “Distress Thermometer” (DT) [28]. In order to identify the potential problems that can induce the distress reported, a problem list (PL) is often added covering the five domains of practical, family, emotional and physical problems, and spiritual/religious concerns.
This study was undertaken to explore the acceptance and feasibility of the DT and PL as a brief screening tool in an ambulatory setting for routine care, as determined by the rate of completed questionnaires. We wanted to elucidate the prevalence of distress and problem areas in melanoma patients. We expected that heightened distress is associated with a higher load of problems. Based on previous studies, we hypothesize distress to be increased in younger, female and single living patients with a more recent diagnosis and under current treatment.
Methods
Participants
The study participants were consecutive melanoma patients attending the Skin Cancer Center of the University of Mainz Medical Center. The inclusion criteria were histologically proven diagnosis of melanoma, age of at least 18 years, the ability to read and understand the questionnaires, and the patient's consent to participate. Using a cross-sectional design, patients were recruited at all stages of disease and treatment during aftercare. Demographic and clinical data were obtained by linking the patient's questionnaire with information stored in the patient's chart. The demographic variables considered were age, gender, employment state, health insurance, and marital state. Clinical data included melanoma stage, time since diagnosis, stage of treatment (pretreatment, current treatment), multiple melanomas, and other cancers.
Procedure
Patients were approached in the waiting areas at the center prior to a scheduled outpatient visit. After an explanation by trained nurses, participants were asked to complete the DT and PL.
Measures
Distress Thermometer and Problem List
The DT is a single-itemed self-reported, pencil and paper measure consisting of a line with a 0–10 scale anchored at the 0 point indicating “no distress” and a scale point 10 indicating “extreme distress”. Patients are instructed to circle the number that best describes the level of distress during the past week. The DT is simple to score and easy to interpret, and since developed in 1998 it has been used and validated in numerous clinical studies and has been recommended as a screening module for distress by the NCCN Panel. In a mixed German sample of cancer patients undergoing rehabilitation, a cut-off score of 5 yielded the best discrimination for high levels of anxiety or depression (based on the HADS) with a sensitivity of 97% and a specificity of 41% [29]. Thus, the internationally recommended cut-off score of 5 was used in this study.
The PL was developed by the Distress Management Guidelines Panel of the NCCN. It consists of 35 problems commonly experienced by cancer patients in five categories (practical problems, family problems, emotional problems, spiritual-religious concerns, and physical problems). Patients indicate whether or not (“yes-no”) they have experienced any of those problems in the past 7 days.
Statistical Analysis
The acceptance and feasibility of the DT and PL as an extremely concise screening tool in an ambulatory setting for routine care of melanoma was determined by the proportion of completed questionnaires. Descriptive statistics were used to characterize the sample with regard to demographic and clinical variables. Following the recommendations of Mehnert et al. [29], we considered a patient as highly distressed when DT ≥5.
To determine the association of DT with demographics and clinical characteristics, we fitted cumulative logit models assuming proportional odds for each variable. This type of model takes into account the ordinal scale of the DT and assumes that odds ratios for each predictor are constant over all possible dichotomizations [30]. It is a generalization of logistic regression to an ordinal outcome.
The association of the DT with PL items was described by fitting proportional odds cumulative logit models for each item, adjusting for demographic variables found to be associated with the DT. To assess the joint influence of PL items on the DT, a proportional odds cumulative logit model was fitted and variables were selected using backward selection. We considered models starting a) with all PL items, b) with the numbers of problems of specific types, and c) the overall number of problems; again we adjusted for demographic variables found to be associated with the DT. The analyses were performed using GraphPad Prism 5.0 and SAS 9.3.
Ethics statement
Approval was received from the Ethic Committee of Rhineland-Pfalz, Germany.
Results
Patient characteristics
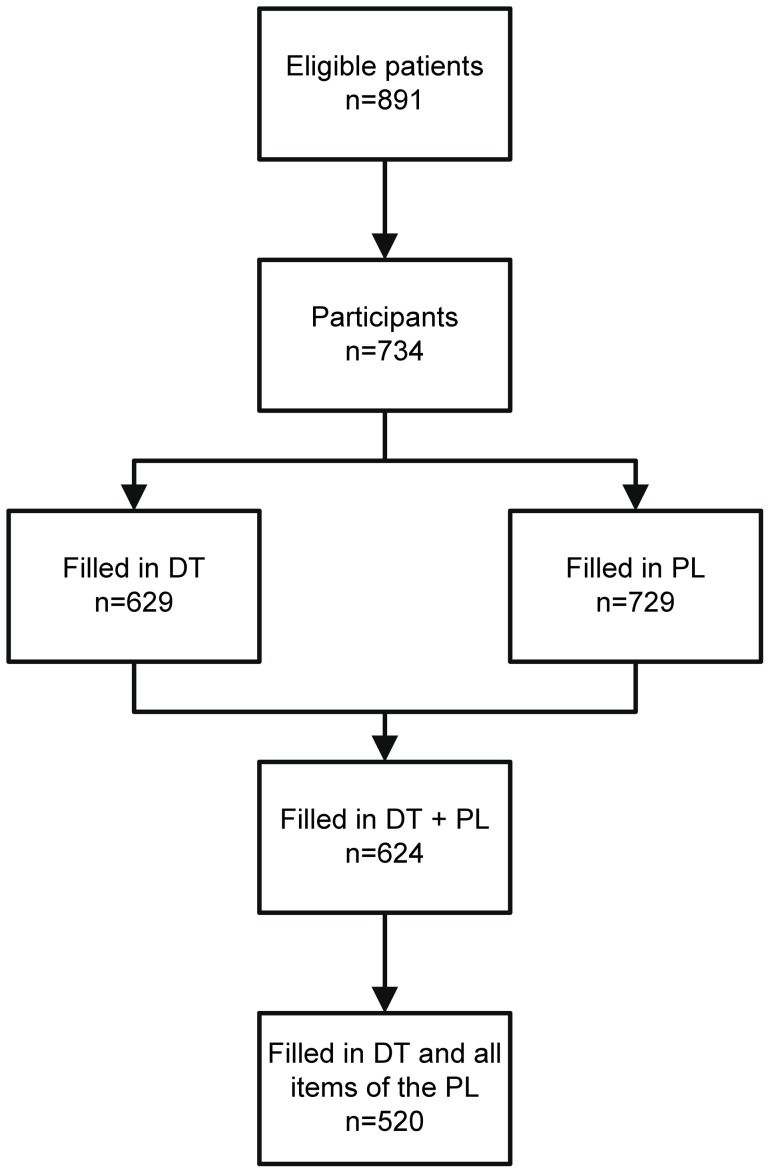
Out of a total of 891 patients with melanoma visiting the skin cancer center during the study accrual period, 734 (82%) patients agreed to participate in the survey. Of these, 629 (86%) patients scored the DT and 729 (99%) patients filled in the PL. Both screening measures were filled in by 624 patients (85%). One or more (on average 3.18) items of the PL were omitted by 147 (20%) of the 729 patients who had filled in the PL. Both the DT and the PL were complete for 520 of 734 recruited patients (71%) (Figure 1). The statistical analysis has been restricted to those 520 patients who completed both the DT and all items of the PL.
Figure 1. Participant flow, details of study recruitment.
The patient characteristics are summarized in Table 1 and Table 2. The average age of the patients included in the analysis was 58.5 (range 18–89, SD 14.0) years. Two hundred forty-three (47%) patients were female, and 277 (53%) were male. Patients with all stages of melanoma according to the classification of the American Joint Committee on Cancer (AJCC) 2009 were included: Melanoma in situ: 26 (5%), stage I/II: 401 (77%), stage III: 69 (13%) and stage IV: 24 (5%).
Table 1. Patient Demography (n = 520).
| Total | ||
| N | % | |
| Sex | ||
| Male | 277 | 53 |
| Female | 243 | 47 |
| Employment status | ||
| Working | 223 | 43 |
| Retired | 171 | 33 |
| Other | 76 | 15 |
| Unknown | 50 | 10 |
| Insurance status | ||
| Public | 431 | 83 |
| Private | 89 | 17 |
| Status of relationship | ||
| Married | 363 | 70 |
| Widowed | 25 | 5 |
| Single (including divorced and separated) | 82 | 16 |
| Unknown | 50 | 10 |
Table 2. Patient clinical variables (n = 520).
| Total | ||
| N | % | |
| AJCC stage | ||
| In situ | 26 | 5 |
| I/II | 401 | 77 |
| III | 69 | 13 |
| IV | 24 | 5 |
| Time since diagnosis | ||
| 0–12 months | 143 | 28 |
| >12–24 months | 81 | 16 |
| >24–36 months | 50 | 10 |
| >36–48 months | 40 | 8 |
| >48–60 months | 44 | 8 |
| >60 months | 162 | 31 |
| SLNB | ||
| No | 361 | 69 |
| Yes | 159 | 31 |
| Lymph node dissection | ||
| No | 452 | 87 |
| Yes | 68 | 13 |
| Surgery for metastases (not skin, not lymph nodes) | ||
| No | 510 | 98 |
| Yes | 10 | 2 |
| Radiotherapy | ||
| No | 508 | 98 |
| Yes | 12 | 2 |
| Type of systemic therapy | ||
| None | 373 | 72 |
| Interferon | 132 | 25 |
| Other1 | 15 | 3 |
| Patient under systemic therapy | ||
| No | 468 | 90 |
| Yes | 52 | 10 |
| Multiple melanomas | ||
| No | 487 | 94 |
| Yes | 33 | 6 |
| Other nonmelanoma skin cancer | ||
| No | 487 | 94 |
| Yes | 33 | 6 |
| Other noncutaneous malignancy | ||
| No | 478 | 92 |
| Yes | 42 | 8 |
Vaccination, Immuntherapy+Vaccination, Chemoimmuntherapy, Chemoimmuntherapy+Vaccination, Chemoimmuntherapy+Targeted Therapy, Interferon+Interleukin-2.
Association between distress score, categorical and continuous variables
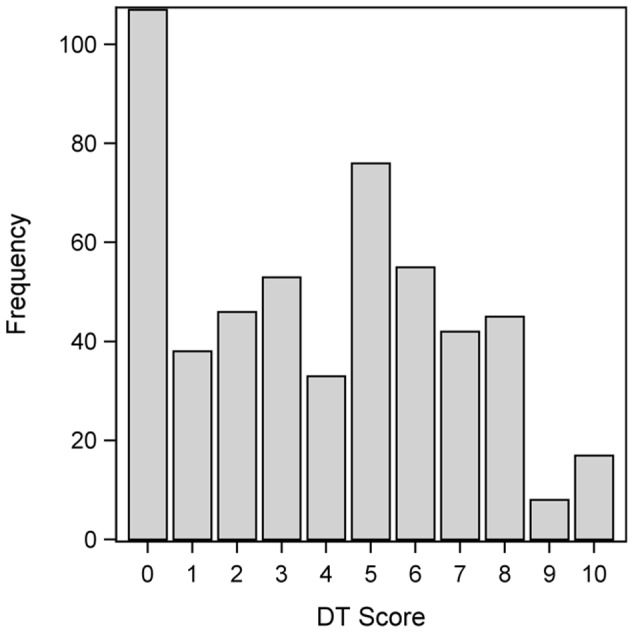
The mean DT score was 3.9 (SD: 3.0), and the median score was 4, with a range from 0 to 10. Figure 2 summarizes the distribution of the DT scores. Two hundred seventy-seven (53%) patients reported a distress score of <5, whereas 243 (47%) scored ≥5 on the DT.
Figure 2. Frequency distribution of DT score among all patients completing the DT and the PL (n = 520).
The association between patient characteristics and distress levels is shown in Table 3. Distress scores show a decreasing trend with age (OR = 0.97 per year, 95% CI [0.96; 0.98]). We found the DT to be associated with employment status, with retired patients having lower DT scores than working patients (OR = 0.51, 95% CI [0.36; 0.72]). Patients under systemic therapy had higher DT scores than patients not currently treated (OR = 1.91, 95% CI [1.16; 3.16]).
Table 3. Association of demographic and clinical variables with high distress (n = 520).
| DT score ≥5 | |||||||
| No | Yes | ||||||
| N | % | N | % | Odds Ratio | 95% confidence interval | ||
| Sex | |||||||
| Male | 153 | 55 | 124 | 45 | 1.0 | ||
| Female | 124 | 51 | 119 | 49 | 1.34 | [0.99; 1.81] | |
| Employment status | |||||||
| Working | 100 | 45 | 123 | 55 | 1.0 | ||
| Retired | 104 | 61 | 67 | 39 | 0.51 | [0.36; 0.72] | |
| Other | 45 | 59 | 31 | 41 | 0.64 | [0.41; 1.01] | |
| Unknown | 28 | 56 | 22 | 44 | 0.9 | [0.52; 1.53] | |
| Insurance status | |||||||
| Public | 225 | 52 | 206 | 48 | 1.0 | ||
| Private | 52 | 58 | 37 | 42 | 0.79 | [0.53; 1.17] | |
| Status of relationship | |||||||
| Married | 192 | 53 | 171 | 47 | 1.0 | ||
| Widowed | 14 | 56 | 11 | 44 | 0.75 | [0.37; 1.54] | |
| Single (including divorced and separated) | 43 | 52 | 39 | 48 | 1.25 | [0.82; 1.89] | |
| Unknown | 28 | 56 | 22 | 44 | 0.86 | [0.51; 1.44] | |
| AJCC stage | |||||||
| In situ | 13 | 50 | 13 | 50 | 1.07 | [0.53; 2.13] | |
| I/II | 219 | 55 | 182 | 45 | 1.0 | ||
| III | 35 | 51 | 34 | 49 | 1.21 | [0.78; 1.89] | |
| IV | 10 | 42 | 14 | 58 | 1.41 | [0.69; 2.89] | |
| SLNB | |||||||
| No | 190 | 53 | 171 | 47 | 1.0 | ||
| Yes | 87 | 55 | 72 | 45 | 0.99 | [ 0.72; 1.37] | |
| Lymph node dissection | |||||||
| No | 241 | 53 | 211 | 47 | 1.0 | ||
| Yes | 36 | 53 | 32 | 47 | 1.14 | [0.73; 1.78] | |
| Surgery for metastases (not skin, not lymph nodes) | |||||||
| No | 272 | 53 | 238 | 47 | 1.0 | ||
| Yes | 5 | 50 | 5 | 50 | 1.03 | [0.35; 3.08] | |
| Radiotherapy | |||||||
| No | 271 | 53 | 237 | 47 | 1.0 | ||
| Yes | 6 | 50 | 6 | 50 | 1.53 | [0.56; 4.15] | |
| Type of systemic therapy | |||||||
| None | 196 | 53 | 177 | 47 | 1.0 | ||
| Interferon | 72 | 55 | 60 | 45 | 1.1 | [0.78; 1.55] | |
| Other1 | 9 | 60 | 6 | 40 | 0.95 | [0.38; 2.33] | |
| Patient under systemic therapy | |||||||
| No | 254 | 54 | 214 | 46 | 1.0 | ||
| Yes | 23 | 44 | 29 | 56 | 1.91 | [1.16; 3.16] | |
| Multiple melanomas | |||||||
| No | 257 | 53 | 230 | 47 | 1.0 | ||
| Yes | 20 | 61 | 13 | 39 | 0.61 | [0.33; 1.14] | |
| Other nonmelanoma skin cancer | |||||||
| No | 259 | 53 | 228 | 47 | 1.0 | ||
| Yes | 18 | 55 | 15 | 45 | 0.77 | [0.41; 1.42] | |
| Other noncutaneous malignancy | |||||||
| No | 254 | 53 | 224 | 47 | 1.0 | ||
| Yes | 23 | 55 | 19 | 45 | 0.98 | [0.56; 1.70] | |
Vaccination, Immuntherapy+Vaccination, Chemoimmuntherapy, Chemoimmuntherapy+Vaccination, Chemoimmuntherapy+Targeted Therapy, Interferon+Interleukin-2.
In order to assess the joint influence of demographics and clinical characteristics, we fitted a multivariate cumulative logit model and found that it was sufficient to include age; adding employment status or therapy status did not improve the model. Other sociodemographic variables such as sex, health insurance, or marital status did not affect distress levels. The detailed results are included in Table 3.
Interestingly, no further association with the distress score was found. In particular, no association exists between disease stage and distress level. Other melanoma-associated parameters such as type of treatment, presence of multiple melanomas, or another cancer diagnosis did not influence distress score, either. Shorter time since diagnosis was not associated with higher distress scores (OR = 1.0 per year, 95% CI [0.96; 1.03]).
Distress score and Problem list
Table 4 provides an overview of the problems that were mentioned most frequently in the problem list, stratified by distress score (high versus low). The strength of association is measured by the odds ratio, adjusted for age.
Table 4. Association of problems indicated with high/low distress (n = 520).
| Problem present | Distress (high vs. low)1 | |||||||||
| No | Yes | |||||||||
| DT score < 5 | DT score ≥ 5 | DT score < 5 | DT score ≥ 5 | |||||||
| Problem | N | % | N | % | N | % | N | % | Odds Ratio | 95% confidence interval |
| At least one problem | 86 | 81 | 20 | 19 | 191 | 46 | 223 | 54 | 6.09 | [4.03; 9.21] |
| Practical problems | ||||||||||
| At least one practical problem | 236 | 62 | 147 | 38 | 41 | 30 | 96 | 70 | 3.08 | [2.14; 4.42] |
| Child care | 273 | 55 | 224 | 45 | 4 | 17 | 19 | 83 | 2.60 | [1.24; 5.46] |
| Housing | 271 | 55 | 226 | 45 | 6 | 26 | 17 | 74 | 3.58 | [1.71; 7.49] |
| Insurance/financial | 264 | 55 | 217 | 45 | 13 | 33 | 26 | 67 | 2.54 | [1.43; 4.53] |
| Mobility/transportation | 264 | 55 | 219 | 45 | 13 | 35 | 24 | 65 | 1.73 | [0.96; 3.10] |
| Work/school | 252 | 58 | 181 | 42 | 25 | 29 | 62 | 71 | 3.25 | [2.12; 4.98] |
| Family problems | ||||||||||
| At least one family problem | 259 | 60 | 175 | 40 | 18 | 21 | 68 | 79 | 4.53 | [2.96; 6.94] |
| Dealing with children | 269 | 55 | 223 | 45 | 8 | 29 | 20 | 71 | 2.15 | [1.10; 4.19] |
| Dealing with partner | 268 | 57 | 205 | 43 | 9 | 19 | 38 | 81 | 5.81 | [3.36; 10.06] |
| Dealing with parents | 269 | 56 | 215 | 44 | 8 | 22 | 28 | 78 | 2.63 | [1.44; 4.80] |
| Emotional problems | ||||||||||
| At least one emotional problem | 180 | 72 | 71 | 28 | 97 | 36 | 172 | 64 | 4.51 | [3.25; 6.25] |
| Depression | 263 | 58 | 193 | 42 | 14 | 22 | 50 | 78 | 4.76 | [2.96; 7.66] |
| Fear | 246 | 62 | 153 | 38 | 31 | 26 | 90 | 74 | 4.33 | [2.97; 6.33] |
| Nervousness | 226 | 64 | 128 | 36 | 51 | 31 | 115 | 69 | 3.85 | [2.74; 5.40] |
| Sadness | 248 | 61 | 159 | 39 | 29 | 26 | 84 | 74 | 4.14 | [2.82; 6.09] |
| Worries | 235 | 65 | 129 | 35 | 42 | 27 | 114 | 73 | 4.19 | [2.92; 6.00] |
| Loss of interest in daily activities | 266 | 55 | 218 | 45 | 11 | 31 | 25 | 69 | 3.95 | [2.16; 7.22] |
| Physical problems | ||||||||||
| At least one physical problem | 119 | 71 | 48 | 29 | 158 | 45 | 195 | 55 | 3.25 | [2.32; 4.55] |
| Appearance | 271 | 56 | 213 | 44 | 6 | 17 | 30 | 83 | 3.12 | [1.70; 5.71] |
| Bathing/dressing | 269 | 54 | 228 | 46 | 8 | 35 | 15 | 65 | 2.06 | [0.99; 4.30] |
| Breathing | 261 | 56 | 204 | 44 | 16 | 29 | 39 | 71 | 2.94 | [1.79; 4.82] |
| Changes in urination | 255 | 54 | 217 | 46 | 22 | 46 | 26 | 54 | 1.64 | [0.97; 2.77] |
| Constipation | 266 | 54 | 224 | 46 | 11 | 37 | 19 | 63 | 2.05 | [1.07; 3.92] |
| Diarrhea | 265 | 54 | 224 | 46 | 12 | 39 | 19 | 61 | 2.04 | [1.08; 3.86] |
| Eating | 253 | 54 | 219 | 46 | 24 | 50 | 24 | 50 | 1.96 | [1.17; 3.33] |
| Fatigue | 212 | 61 | 135 | 39 | 65 | 38 | 108 | 62 | 2.29 | [1.65; 3.18] |
| Feeling swollen | 248 | 55 | 205 | 45 | 29 | 43 | 38 | 57 | 1.90 | [1.21; 2.99] |
| Fevers | 273 | 53 | 240 | 47 | 4 | 57 | 3 | 43 | 0.70 | [0.19; 2.61] |
| Getting around | 260 | 56 | 207 | 44 | 17 | 32 | 36 | 68 | 3.47 | [2.09; 5.76] |
| Indigestion | 264 | 55 | 218 | 45 | 13 | 34 | 25 | 66 | 1.88 | [1.05; 3.35] |
| Mouth sores | 262 | 54 | 223 | 46 | 15 | 43 | 20 | 57 | 2.28 | [1.25; 4.17] |
| Nausea | 269 | 55 | 219 | 45 | 8 | 25 | 24 | 75 | 3.16 | [1.67; 5.95] |
| Nose dry/congested | 245 | 56 | 196 | 44 | 32 | 41 | 47 | 59 | 1.75 | [1.15; 2.66] |
| Pain | 247 | 60 | 164 | 40 | 30 | 28 | 79 | 72 | 3.31 | [2.26; 4.85] |
| Sexual | 255 | 55 | 209 | 45 | 22 | 39 | 34 | 61 | 1.85 | [1.14; 3.01] |
| Sleep | 221 | 59 | 153 | 41 | 56 | 38 | 90 | 62 | 2.02 | [1.43; 2.83] |
| Tingling in hands/feet | 234 | 57 | 180 | 43 | 43 | 41 | 63 | 59 | 1.70 | [1.17; 2.47] |
| Spiritual problems | 274 | 54 | 238 | 46 | 3 | 38 | 5 | 63 | 4.52 | [1.32; 15.47] |
| Other problems | 259 | 55 | 210 | 45 | 18 | 35 | 33 | 65 | 2.34 | [1.41; 3.90] |
The odds ratios and corresponding 95% confidence intervals in this table are obtained by fitting a proportional odds cumulative logit model for each problem list item, adjusting for age. The odds ratios given here describe the odds for high distress in the presence of a problem relative to the odds in the absence of the problem.
Most patients mentioned at least one physical problem (n = 353; 68%), and at least one emotional problem was reported by 269 (52%) patients. Practical problems were mentioned by 137 (26%), family problems were indicated by 86 (17%) patients, and other problems by 51 (10%) patients. Spiritual problems were rarely mentioned (n = 8; 1.5%).
Patients reporting more problems were more likely to score highly on the DT. This applied to emotional problems in general (OR = 4.51, 95% CI [3.25; 6.25]) and to each emotional problem in particular (nervousness (OR = 3.85, 95% CI [2.74; 5.40]), worries (OR = 4.19, 95% CI [2.92; 6.00]), fear (OR = 4.33, 95% CI [2.97; 6.33]), sadness (OR = 4.14, 95% CI [2.82; 6.09]), depression (OR = 4.76, 95% CI [2.96; 7.66]), and loss of interest in daily activities (OR = 3.95, 95% CI [2.16; 7.22]). This was also true for family problems in general (OR = 4.53, 95% CI [2.96; 6.94]) and for each family problem in particular (Table 4). The most pronounced association of a family problem with the DT was observed in “dealing with partner” (OR = 5.81, 95% CI [3.36; 10.06]). In practical problems, work/school problems and problems with child care were associated strongly with high distress scores. Problems with housing also showed a strong association with the DT, but were observed only in 23 patients (4.2%).
In general, physical problems were also associated with high DT scores (OR = 3.25, 95%CI [2.32; 4.55]); the most pronounced associations were observed in problems with pain (OR = 3.31, 95% CI [2.26; 4.85]), appearance (OR = 3.12, 95% CI [1.70; 5.71]), getting around (OR = 3.47, 95% CI [2.09; 5.76]) and nausea (OR = 3.16, 95% CI [1.67; 5.95]). Spiritual problems showed more than a random association with high DT scores (OR = 4.52, 95% CI [1.32; 15.47]; however, they were rare in our patients. Fevers, changes in urination and problems with bathing/dressing did not show any association with DT scores.
As expected, distress scores were associated with the number of problems indicated by patients (OR = 1.24 per problem, 95%CI [1.19; 1.29]; i.e. patients with multiple problems had higher distress scores (Figure 3).
Figure 3. Joint distribution of DT scores and number of problems.
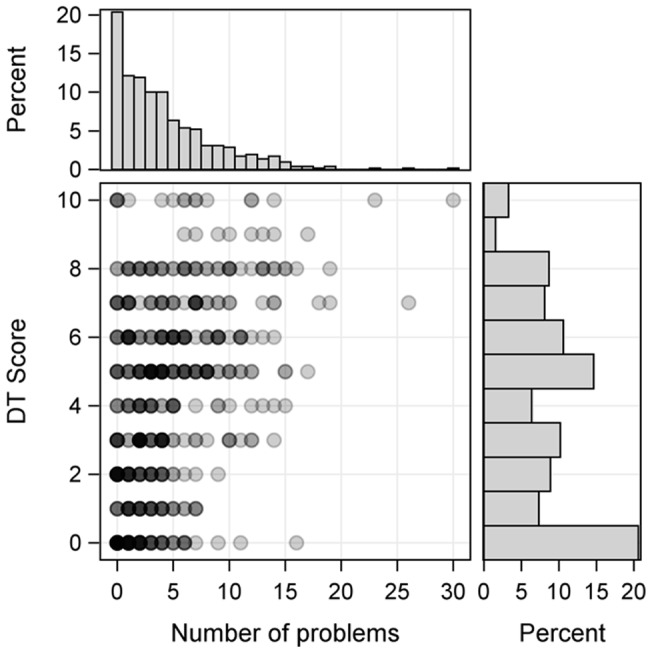
The scatter plot (bottom left) depicts the association of the DT score and number of problems. The darkness of the dots indicates the number of patients having a particular combination of number of problems and the DT score. Dark shades correspond to many patients, light shades correspond to few patients. The histograms for the number of problems (top left) and for the DT score (bottom right) show the marginal distributions of these two traits.
We modeled the joint influence of problems on the DT using a cumulative logit model, adjusting for age and applying backward selection. The first considered model started with all problem list items. Work/education related problems, problems dealing with partner, fear, nervousness, worries, breathing problems and pain remained in the selected model. When this model was fitted, we found 72% concordant pairs. The model selected when starting with the numbers of specific problems, i.e. practical, emotional, physical, spiritual, and family problems, contained the number of practical problems (OR = 1.35 per problem, 95% CI [1.10; 1.67], number of emotional problems (OR = 1.66 per problems; 95% CI [1.48; 1.87], and number of physical problems (OR = 1.95 per problem, 95% CI [1.37; 2.78]); here we found 71.7% concordant pairs. When only considering the overall number of problems, we found similarly good classification properties: 70.4% concordant pairs.
Discussion
In general melanoma patients seem to be highly motivated to participate in psycho-oncological screening programs as 82% of the patients agreed to participate in this survey. Of the patients willing to participate, 71% filled in both screening measures, the DT and the PL, completely. Compared to the literature, which reports DT participation rates of about 80% to 90% [31], [32], our data are consistent. In the studies where DT with PL were combined, there are no clear data regarding the completeness of the PL, as often imputations were made for missing data [32].To our knowledge we have presented this kind of data for the first time in detail within a patient collective of 734 participating patients. We found a high motivation to participate in general as well as to fill in both the DT and the PL screening measures. However, there were some problems in filling in the complete survey with missing data in 29% of participating patients (average 3.18 missing items per patient). We have no information on why those patients skipped some items as we could not find any pattern of missing single items. Potential reasons could be that patients found some items not applicable for themselves or not important enough to mention.
The mean distress score in our melanoma patients was found to be 3.9, with 47% of patients reporting distress intensity between 5 and 10 on the DT. The mean score and percentages fell in the middle range reported in previous studies with ambulatory care cancer patients (mean of DT score of previous studies: 2.47–4.7) [31], [33]–[35].
In our sample, elevated distress scores were not associated with most disease-specific melanoma aspects such as previous treatment, localization of the tumor, tumor stage, or time since diagnosis. Only current treatment was associated with an increased distress, which is supported by the literature; a considerable number of our patients received adjuvant interferon alpha treatment, a treatment known to affect several aspects of quality of life [36], [37]. Apart from younger age and employment state, there was no association of distress with demographic data, including gender, health insurance, or marital state. This finding could indicate that melanoma patients are not strongly distressed due to their disease. Indeed, compared to other cancer entities, long-time melanoma survivors as well as prostate cancer patients seem to perform better [27]. To assess the impact of melanoma-specific distress, studies including distress data from the general population should be initiated.
Melanoma patients differ from other cancer patients in several aspects. On average, melanoma patients are younger than other cancer patients. This implies that diagnosis usually occurs at a time when most patients are still active at work, have to care for their children or pay off their mortgage. Being confronted with a possibly life threatening disease in a period of life characterized by career and family duties may cause existential concerns, particularly in younger patients [1], [38].
Most melanoma patients are diagnosed at an early stage of the disease without the need for further adjuvant treatment after initial surgery. The absence of any signs of disease together with a lack of physical impairment may distract the patient from the cancer diagnosis and may enable the patient to cope well with his or her disease. Indeed, most patients with melanoma seem to cope well; however even patients with early-stage disease have to deal with the possibility of recurrence or systemic spread, which is highest in the first 3 years but can also occur more than 10 years after diagnosis. This underlying fear could explain the distress of our patients regardless of disease stage, suggesting that the possibility of disease recurrence/metastasis is the major stress factor.
Accordingly, the most prevalent problems of our patients who had distress scores indicating psychosocial referral were of emotional nature. Consistent with published data [39], in addition to other emotional problems worries and fear were strongly associated with high distress. In a recent survey of 1490 cancer patients, Mergenthaler et al. [31] found that 97% of patients appreciated speaking with their doctor about their distress, and 56% felt better than usual after this consultation. As distress in our patients was not only associated with emotional problems but also with practical, family, and physical problems like dealing with their partner, problems at work, nausea, or pain, the role of the primary physician to meet and treat unmet needs should not be underestimated. The DT with the PL can help identify distress sources and stimulate doctor-patient communication. The primary physician than can act as a gate keeper, who refers the patient to the specific professional he or she needs: for physical problems to physicians or nurses, for emotional problem to psycho-oncologists, and for practical problems to social workers.
There are certain limitations in our study that should be considered. First, it has a cross-sectional design. A longitudinal study could probably better address patients' needs during their disease process and identify patients in need for professional psychosocial support. Second, DT and PL have only been studied in cancer patients but not in the general population, and third, we did not investigate the influence of comorbidities, especially chronic diseases other than cancer and mental illnesses. Therefore the impact of melanoma disease on distress still has to be defined in further studies considering these issues.
Conclusions
The present findings have important implications for future research and clinical practice. First, the results suggest that the DT and PL may be used to identify distressed melanoma patients. It seems to be an economically reasonable initial measure and helps to better identify patients who would actually profit from further psychosocial intervention. Even though melanoma patients regardless of stage seem to cope well with their disease, younger patients who are currently employed and patients under current systemic treatment should be followed more cautiously. As distress can be influenced not only by disease-specific items but also by problems of daily living, comorbidities, a patient's history, or socioeconomic issues, a patients' concerns depicted by the DT and PL should stimulate doctor-patient communication and help to guide the patient to psychosocial professionals according to the patient's needs.
Funding Statement
The authors have no support or funding to report.
References
- 1.National Comprehensive Cancer Network (2012) NCCN Distress Management Clinical Practice Guidelines in Oncology. Available: http://www.nccn.org. Accessed 2013 Apr 12.
- 2. Holland JC (1999) NCCN practice guidelines for the management of psychosocial distress. Oncology 13: 113–147. [PubMed] [Google Scholar]
- 3.Simard EP, Ward EM, Siegel R, Jemal A (2012) Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin [Epub ahead of print]. [DOI] [PubMed]
- 4.AWMF online (2013) S3-guideline malignant melanoma. Available: http://www.awmf.org. Accessed 2013 Apr 12.
- 5.Kasparian NA, Sansom-Daly U, McDonald RP, Meiser B, Butow PN, et al.. (2011) The nature and structure of psychological distress in people at high risk melanoma: A factor analytic study. Psychooncology [Epub ahead of print]. [DOI] [PubMed]
- 6.Atkinson TM, Noce NS, Hay J, Rafferty BT, Brady MS (2012) Illness-related distress in women with clinically localized cutaneous melanoma. Ann Surg Oncol [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 7.Roberts N, Czajkowska Z, Radiotis G, Körner A (2012) Distress and coping strategies among patients with skin cancer. J Clin Psychol Med Settings [Epub ahead of print]. [DOI] [PubMed]
- 8. Kennard BD, Smith SM, Olvera R, Bawdon RE, O'hAilin A, et al. (2004) Nonadherence in adolescent oncology patients: preliminary data on psychological risk factors and relationships to outcome. J Clin Psychol Med Settings 11: 30–39. [Google Scholar]
- 9. Pelletier G, Verhoef MJ, Khatri N, Hagen N (2002) Quality of life in brain tumor patients: the relative contributions of depression, fatigue, emotional distress, and existential issues. J Neurooncol 57: 41–49. [DOI] [PubMed] [Google Scholar]
- 10. Skarstein J, Aass N, Fosså SD, Skovlund E, Dahl AA (2000) Anxiety and depression in cancer patients: relation between the Hospital Anxiety and Depression Scale and the European organisation of research and treatment of cancer core quality of life questionnaire. J Psychosom Res 49: 27–34. [DOI] [PubMed] [Google Scholar]
- 11. Stark D, Kiely M, Smith A, Velikova G, House A, et al. (2002) Anxiety disorders in cancer patients: Their nature, associations, and relation to quality of life. J Clin Oncol 20: 3137–3148. [DOI] [PubMed] [Google Scholar]
- 12. Kneier AW (2003) Coping with melanoma: ten strategies that promote psychological adjustment. Surg Clin North Am 83: 417–430. [DOI] [PubMed] [Google Scholar]
- 13. Mosher CE, DuHamel KN, Rini CM, Li Y, Isola L, et al. (2010) Barriers to mental health service use among hematopoietic SCT survivors. Bone Marrow Transplant 45: 570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown JE, Butow PN, Culjak G, Coates AS, Dunn SM (2000) Psychosocial predictors of outcome: time to relapse and survival in patients with early stage melanoma. Br J Cancer 83: 1448–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Letho US, Ojanen M, Dyba T, Aromaa A, Kellokumpu-Lethinen P (2007) Baseline psychosocial predictors of survival in localized melanoma. J Psychosom Res 63: 9–15. [DOI] [PubMed] [Google Scholar]
- 16. Prieto JM, Atala J, Blanch J, Carreras E, Rovira M, et al. (2005) Role of depression as a predictor of mortality among cancer patients after stem-cell transplantation. J Clin Oncol 23: 6063–6071. [DOI] [PubMed] [Google Scholar]
- 17. Prieto JM, Blanch J, Atala J, Carreras E, Rovira M, et al. (2002) Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem-cell transplantation. J Clin Oncol 20: 1907–1917. [DOI] [PubMed] [Google Scholar]
- 18. Bultz BD, Carlson LE (2005) Emotional distress: the sixth vital sign in cancer care. J Clin Oncol 23: 6440–6441. [DOI] [PubMed] [Google Scholar]
- 19. Ollerton I (1995) Class barriers to psychotherapy and counselling. J Psychiatr Ment Health Nurs 2: 91–95. [DOI] [PubMed] [Google Scholar]
- 20. Peppercorn JM, Smith TJ, Helft PR, Debono DJ, Berry SR, et al. (2011) American Society of Clinical Oncology. American society of clinical oncology statement: toward individualized care for patients with advanced cancer. J Clin Oncol 29: 755–760. [DOI] [PubMed] [Google Scholar]
- 21. McDonald MV, Passik SD, Dugan W, Rosenfeld B, Theobald DE, et al. (1999) Nurses' recognition of depression in their patients with cancer. Oncol Nurs 26: 593–599. [PubMed] [Google Scholar]
- 22. Söllner W, DeVries A, Steixner E, Luka P, Sprinzl G, et al. (2001) How successful are oncologists in identifying patients distress, perceived social support, and need for psychosocial counselling? Br J Cancer 84: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.German Cancer Society (2010) Erhebungsbogen für Hautkrebszentren. Available: http://www.krebsgesellschaft.de/download/eb_haut-d203.12.2010.doc. Accessed 2013 Apr 12.
- 24. Newton-Bishop JA, Nolan C, Turner F, McCabe M, Boxer C, et al. (2004) A quality–of-life study in high-risk (thickness ≥2 mm) cutaneous melanoma patients in a randomized trial of 1-cm versus 3-cm surgical excision margins. J Investig Dermatol Symp Proc 9: 152–159. [DOI] [PubMed] [Google Scholar]
- 25. Task PC, Paterson AG, Hayasaka S, Dunn RL, Riba M, et al. (2001) Psychosocial characteristics of individuals with non-stage IV melanoma. J Clin Oncol 19: 2844–2850. [DOI] [PubMed] [Google Scholar]
- 26. Kasparian NA, McLoone JK, Butow PN (2009) Psychological responses and coping strategies among patients with malignant melanoma: a systematic review of the literature. Arch Dermatol 145: 1415–1427. [DOI] [PubMed] [Google Scholar]
- 27. Beutel ME, Blettner M, Fischbeck S, Loquai C, Werner A, et al. (2009) Psycho-oncological aspects of malignant melanoma. A systematic review from 1990–2008. Hautarzt 60: 727–733. [DOI] [PubMed] [Google Scholar]
- 28. Roth AJ, Kornblith AB, Batel-Copel L, Peabody E, Scher HI, et al. (1998) Rapid screening for psychological distress in men with prostate carcinoma: a pilot study. Cancer 82: 1904–1908. [DOI] [PubMed] [Google Scholar]
- 29. Mehnert A, Müller D, Lehmann C, Koch U (2006) The German Version of the NCCN Distress Thermometer: Validation of a Screening Instrument for Assessment of Psychosocial Distress in Cancer Patient. ZPPP 54: 213–223. [Google Scholar]
- 30.Agresti A (2007) An Introduction to categorical data analysis 2nd edition. Hoboken: Wiley. p. 372.
- 31. Mergenthaler U, Heymanns J, Köppler H, Thomalla J, van Roye C, et al. (2011) Evaluation of psychosocial distress in patients treated in a community-based oncology group practice in Germany. Ann Oncol 22: 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mertz B, Bistrup P, Johansen C, Dalton S, Deltour I, et al. (2012) Psychological distress among women with newly diagnosed breast cancer. Eur J Oncol Nurs 16: 439–43. [DOI] [PubMed] [Google Scholar]
- 33. Kendall J, Glaze K, Oakland S, Hansen J, Parry C (2011) What do 1271 distress screeners tell us about cancer patients in a community cancer centre? Psycho-Oncology 20: 594–600. [DOI] [PubMed] [Google Scholar]
- 34. Jacobsen PB, Donovan KA, Trask PC, Fleishman SB, Zabora J, et al. (2005) Screening for psychologic distress in ambulatory cancer patients. Cancer 103: 1494–1502. [DOI] [PubMed] [Google Scholar]
- 35. Tuinman MA, Gazendam-Donofrio SM, Hoekstra-Weebers JE (2008) Screening and referral for psychosocial distress in oncologic practice. Cancer 113: 870–878. [DOI] [PubMed] [Google Scholar]
- 36. Loquai C, Nashan D, Hensen P, Luger TA, Grabbe S, et al. (2008) Safety of pegylated interferon-alpha-2a in adjuvant therapy of intermediate and high-risk melanomas. Eur J Dermatol 18: 29–35. [DOI] [PubMed] [Google Scholar]
- 37. Ziefle S, Egberts F, Heinze S, Volkenandt M, Schmid-Wendtner M, et al. (2011) Health-related quality of life before and during adjuvant interferon-α treatment for patients with malignant melanoma (DeCOG-Trial). J Immunother 34: 403–408. [DOI] [PubMed] [Google Scholar]
- 38. Graves KD, Arnold SM, Love CL, Kirsh KL, Moore PG, et al. (2007) Distress screening in a multidisciplinary lung cancer clinic: prevalence and predictors of clinically significant distress. Lung Cancer 55: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blum A, Blum D, Stroebel W, Rassner G, Garbe C, et al. (2003) Psychosocial burden and subjective experience of melanoma patients in the ambulant follow-up. Psychother Psychosom Med Psychol 53: 258–266. [DOI] [PubMed] [Google Scholar]