Abstract
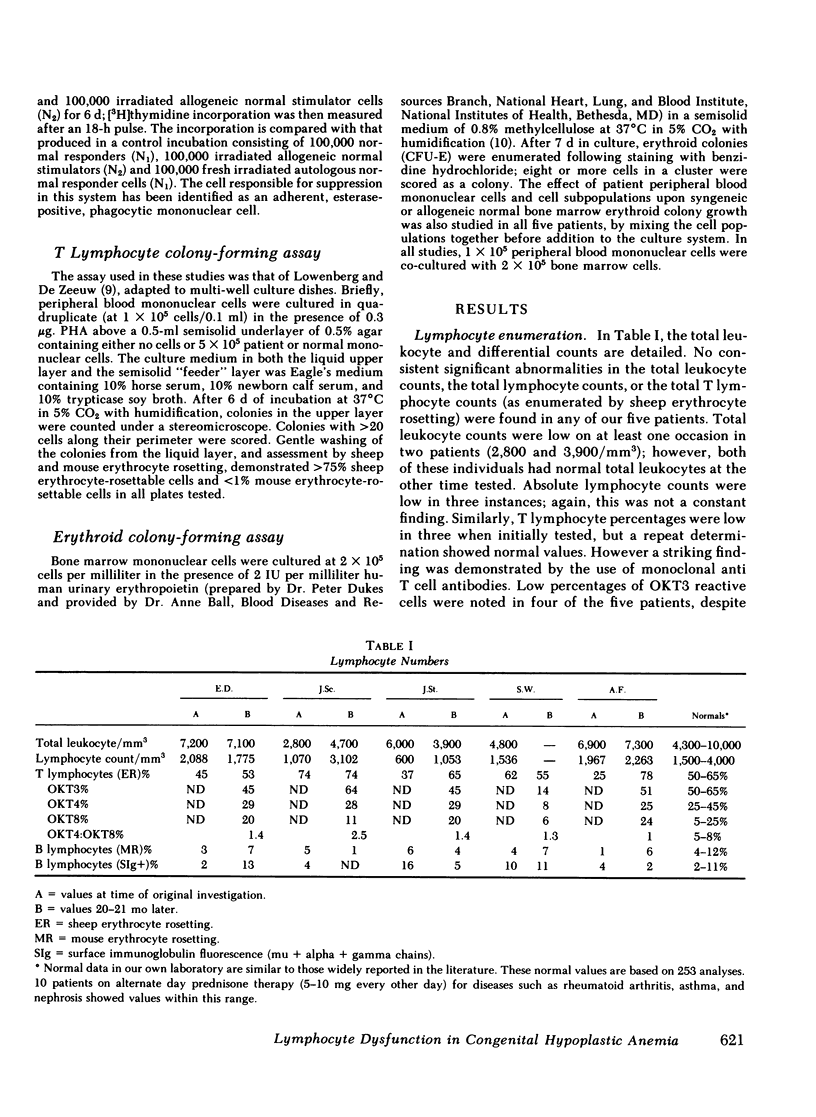
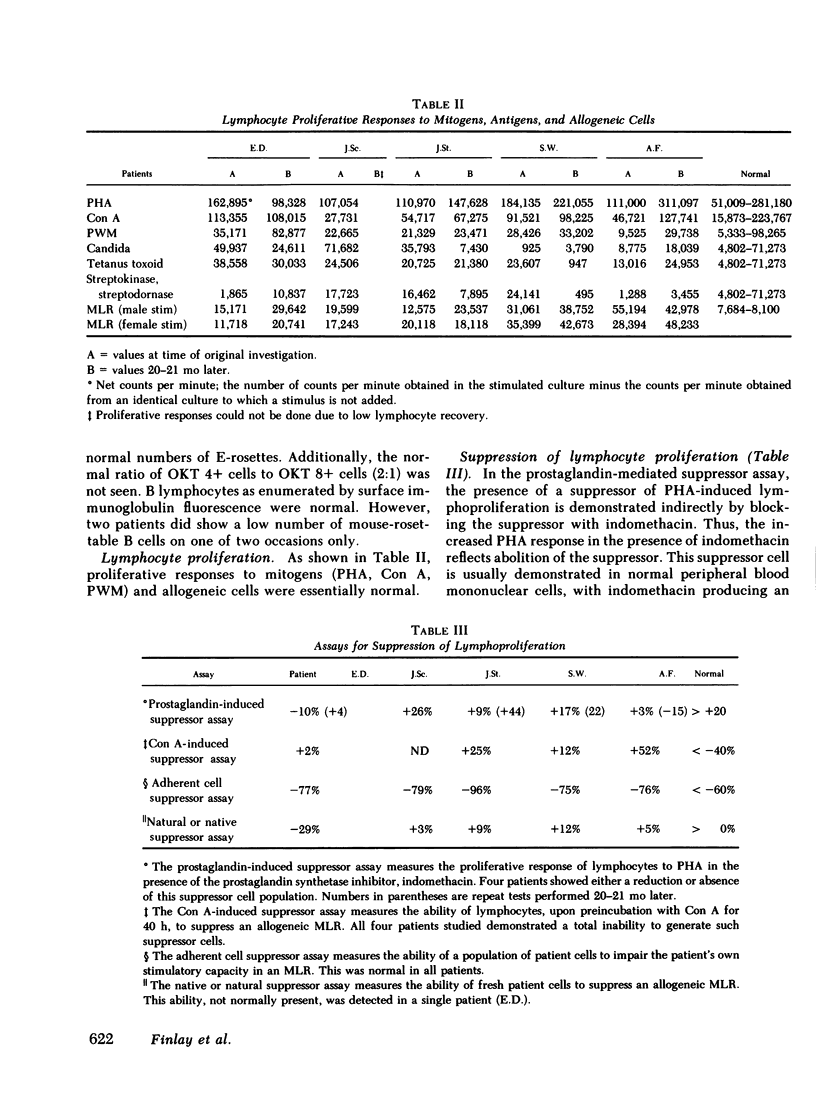
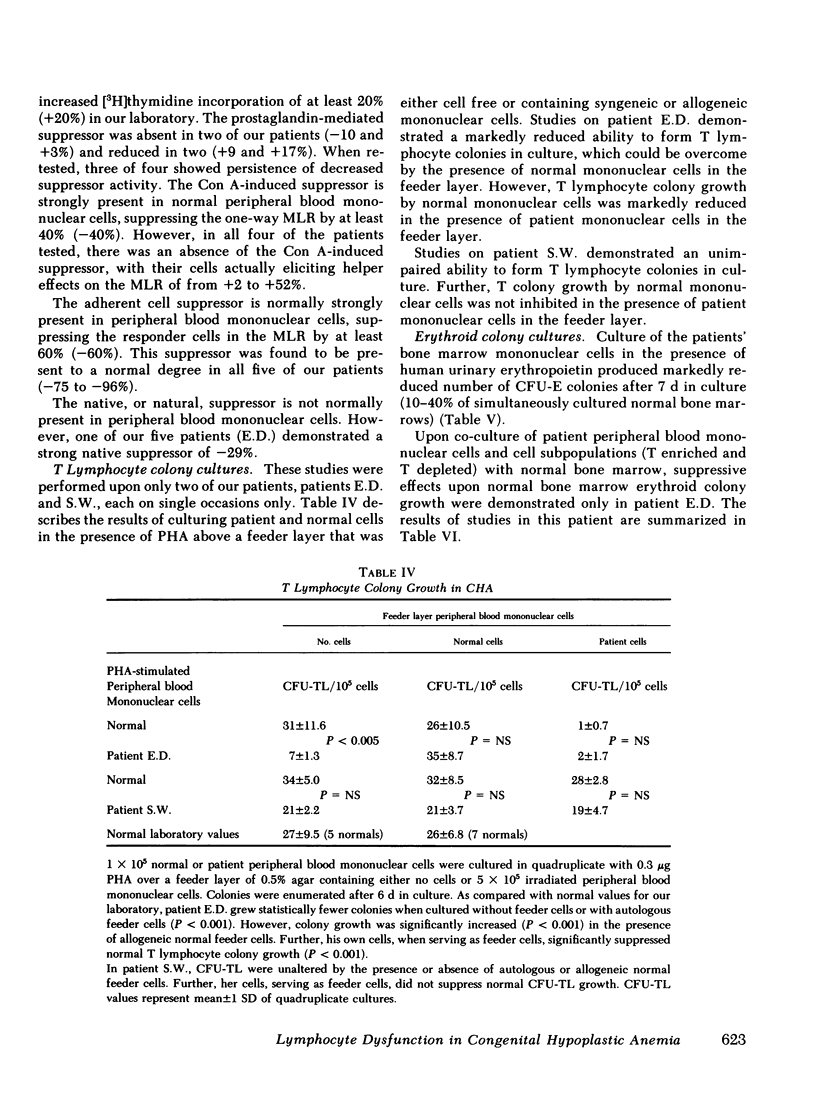
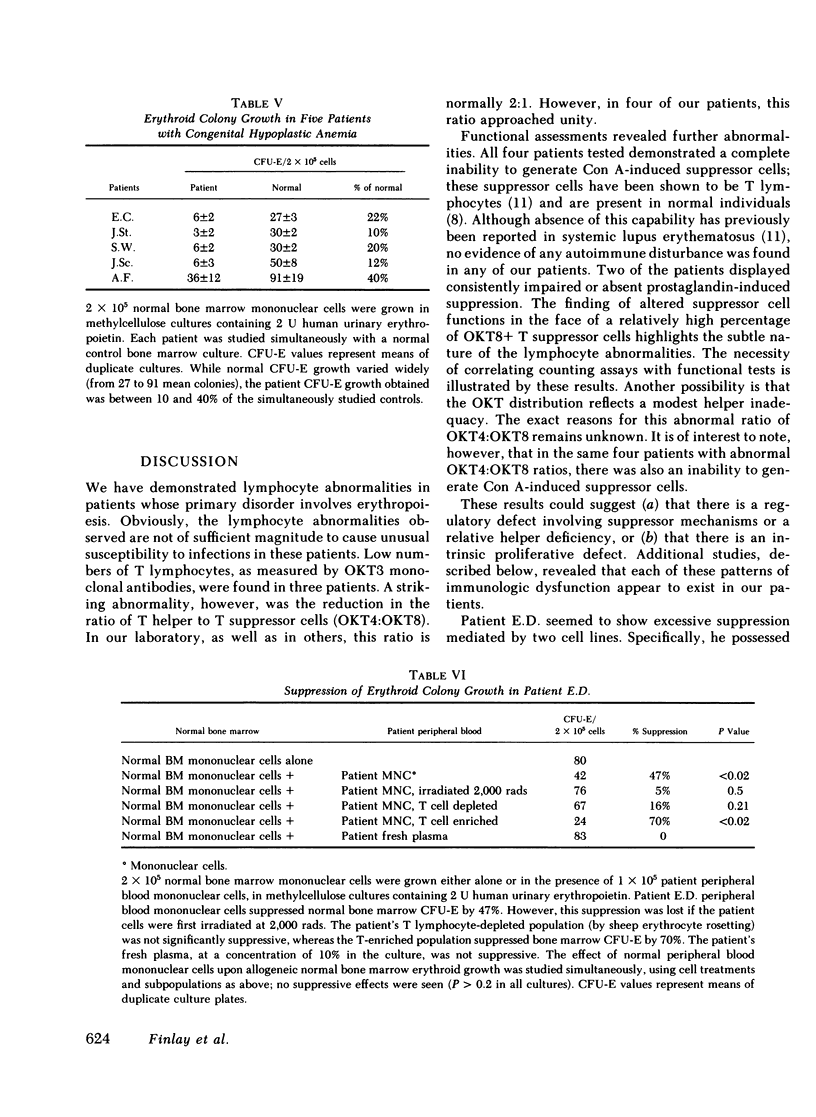
Congenital hypoplastic anemia (Diamond-Blackfan syndrome) is thought to involve the erythropoietic cell line alone. In this study, the evaluation of lymphocyte function in five patients with this syndrome revealed a number of abnormalities. Peripheral blood T lymphocyte percentages as assessed by monoclonal antibodies were decreased in three patients. T-helper/T-suppressor cell (OKT4:OKT8) ratios were almost unity in four of the five patients. We usually find a ratio of 2:1 in normal populations. Studies of lymphocyte-mediated suppression of lymphoproliferation demonstrated an inability to generate concanavalin A-induced suppressor cells in the same four patients and impaired prostaglandin-mediated suppression in two patients. Co-culture studies revealed a T lymphocyte-mediated suppression of erythropoiesis in a single patient, who also showed suppression of the mixed lymphocyte reaction. The four remaining patients showed no excessive suppressor effects either upon erythropoiesis or lymphoproliferation. These studies demonstrate that in congenital hypoplastic anemia, the cellular defect is not restricted to the erythroid progenitor cells, but extends to the lymphocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Diamond L. K., Wang W. C., Alter B. P. Congenital hypoplastic anemia. Adv Pediatr. 1976;22:349–378. [PubMed] [Google Scholar]
- Freedman M. H., Saunders E. F. Diamond-Blackfan syndrome: evidence against cell-mediated erythropoietic suppression. Blood. 1978 Jun;51(6):1125–1128. [PubMed] [Google Scholar]
- Hoffman R., Zanjani E. D., Vila J., Zalusky R., Lutton J. D., Wasserman L. R. Diamond-Blackfan syndrome: lymphocyte-mediated suppression of erythropoiesis. Science. 1976 Sep 3;193(4256):899–900. doi: 10.1126/science.986086. [DOI] [PubMed] [Google Scholar]
- Horowitz S., Borcherding W., Moorthy A. V., Chesney R., Schulte-Wisserman H., Hong R. Induction of suppressor T cells in systemic lupus erythematosus by thymosin and cultured thymic epithelium. Science. 1977 Sep 2;197(4307):999–1001. doi: 10.1126/science.302032. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Ito K., Sakura N., Usui T., Uchino H. Screening for primary immunodeficiencies associated with purine nucleoside phosphorylase deficiency or adenosine deaminase deficiency. J Lab Clin Med. 1977 Nov;90(5):844–848. [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton J. M., Reinherz E. L., Kudisch M., Jackson P. L., Schlossman S. F., Nathan D. G. Mature bone marrow erythroid burst-forming units do not require T cells for induction of erythropoietin-dependent differentiation. J Exp Med. 1980 Aug 1;152(2):350–360. doi: 10.1084/jem.152.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwenberg B., De Zeeuw H. M. A method for cloning T-lymphocytic precursors in agar. Am J Hematol. 1979;6(1):35–43. doi: 10.1002/ajh.2830060106. [DOI] [PubMed] [Google Scholar]
- Nathan D. G., Chess L., Hillman D. G., Clarke B., Breard J., Merler E., Housman D. E. Human erythroid burst-forming unit: T-cell requirement for proliferation in vitro. J Exp Med. 1978 Feb 1;147(2):324–339. doi: 10.1084/jem.147.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D. G., Hillman D. G., Chess L., Alter B. P., Clarke B. J., Breard J., Housman D. E. Normal erythropoietic helper T cells in congenital hypoplastic (Diamond-Blackfan) anemia. N Engl J Med. 1978 May 11;298(19):1049–1051. doi: 10.1056/NEJM197805112981903. [DOI] [PubMed] [Google Scholar]
- Parkman R. The immunopathology of marrow failure. Clin Haematol. 1978 Oct;7(3):475–486. [PubMed] [Google Scholar]
- Potter M. R., Moore M. Characterisation and separation of human lymphocytes forming mouse red cell rosettes. J Immunol Methods. 1978;19(2-3):125–135. doi: 10.1016/0022-1759(78)90173-4. [DOI] [PubMed] [Google Scholar]
- Rice L., Laughter A. H., Twomey J. J. Three suppressor systems in human blood that modulate lymphoproliferation. J Immunol. 1979 Mar;122(3):991–996. [PubMed] [Google Scholar]
- Rinehart J. J., Zanjani E. D., Nomdedeu B., Gormus B. J., Kaplan M. E. Cell-cell interaction in erythropoiesis. Role of human monocytes. J Clin Invest. 1978 Nov;62(5):979–986. doi: 10.1172/JCI109227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. H., Coleman M. F., Pennebaker J. B. Diamond-Blackfan syndrome: evidence for T-cell mediated suppression of erythroid development and a serum blocking factor associated with remission. Br J Haematol. 1979 Jan;41(1):57–68. doi: 10.1111/j.1365-2141.1979.tb03681.x. [DOI] [PubMed] [Google Scholar]
- Winchester R. J., Fu S. M., Hoffman T., Kunkel H. G. IgG on lymphocyte surfaces; technical problems and the significance of a third cell population. J Immunol. 1975 Apr;114(4):1210–1212. [PubMed] [Google Scholar]


