Abstract
Purpose
Weight regain after gastric bypass (GBP) can be associated with a gastrogastric fistula (GGF), in which a channel forms between the gastric pouch and gastric remnant, allowing nutrients to pass through the ‘old route’ rather than bypassing the duodenum. To further understand the mechanisms by which GGF may lead to weight regain, we investigated gut hormone levels in GBP patients with a GGF, before and after repair.
Materials and Methods
Seven post-GBP subjects diagnosed with GGF were studied before and 4 months after GGF repair. Another cohort of 22 GBP control subjects without GGF complication were studied before and 1 year post-GBP. All subjects underwent a 50g oral glucose tolerance test and blood was collected from 0-120minutes for glucose, insulin, ghrelin, PYY3-36, GIP, and GLP-1 levels.
Results
Four months after GGF repair subjects lost 6.0 ±3.9kg and had significantly increased postprandial PYY3-36 levels. After GGF repair, fasting and postprandial ghrelin levels decreased and were strongly correlated with weight loss. The insulin response to glucose also tended to be increased after GGF repair, however no concomitant increase in GLP-1 was observed. Compared to the post-GBP group, GLP-1 and PYY3-36 levels were significantly lower before GGF repair; however, after GGF repair, PYY3-36 levels were no longer lower than the post-GBP group.
Conclusions
These data utilize the GGF model to highlight the possible role of duodenal shunting as a mechanism of sustained weight loss after GBP, and lend support to the potential link between blunted satiety peptide release and weight regain.
Keywords: gastric bypass, gastrogastric fistula, GLP-1, PYY3-36, ghrelin, weight loss
Introduction
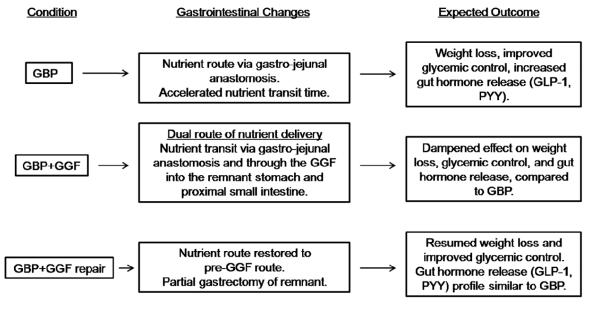
Roux-en-Y gastric bypass surgery (GBP) has been reported to result in an approximate 30% weight loss with improvement and/or remission of obesity related co-morbidities, such as type 2 diabetes (T2DM), in approximately 40-80% of cases [1,2]. Weight regain has been described after GBP in approximately 20-35 % of cases, generally 2 years or more following surgery [3]. This weight regain can at times be associated with the formation of a gastrogastric fistula (GGF), a complication in which a channel forms between the gastric pouch and gastric remnant, allowing nutrients to pass through the remnant and proximal intestine (ie-‘old route’), rather than bypassing these, as is observed with GBP [4] (Figure 1).
Fig. 1.
Gastrointestinal changes and expected metabolic and hormonal changes after GBP, GBP with a GGF, and GBP with a repaired GGF.
The mechanism of sustained weight loss after GBP is not fully understood, however, enhanced release of gut satiety peptides is implicated. The shunting of the proximal small intestine (i.e.-foregut hypothesis) and accelerated nutrient transit to the distal small intestine (i.e.-hindgut hypothesis) both appear to contribute to the enhanced release of satiety peptides such as peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) after GBP [5-8]. In addition, the absence of a rise in circulating levels of the orexigenic hormone ghrelin after GBP, observed in the early few months after the surgery [9], could explain the additional benefit of decreased food intake and weight loss early after GBP. GGF, once a fairly common occurrence after open GBP surgery, occurs now more rarely, at a rate of about 1-2% due to the use of improved bariatric laparoscopic techniques [10].
Although GGF is no longer a major post-operative concern, it presents a rare opportunity to study the endocrine mechanisms of weight gain and/or diabetes relapse in post-GBP patients. We hypothesized that subjects who developed a GGF would have a pattern of gut peptide release similar to that of pre-GBP patients, while the post-GGF repair condition would be similar to the post-GBP state with respect to weight loss, improved glucose homeostasis and pattern of gut hormone release.
Materials and Methods
Subjects
A total of 31 patients participated in this study. Nine patients, diagnosed with GGF post-GBP, enrolled in the GGF COHORT. One patient could not be contacted after GGF repair and another had poor IV access, therefore only 7 women from this cohort completed the study, two of whom had T2DM. The diagnosis of GGF was documented by upper endoscopy and/or Gastrografin studies by the surgical team.
Twenty-two participants (2 men and 20 women) with successful weight loss and no complications were studied before and 1 year after GBP and served as CONTROLS.
The study was approved by the St. Luke’s-Roosevelt Hospital Center Institutional Review Board and all participants provided written informed consent prior to enrollment in the study.
Study Design
Surgical procedures
GBP surgery
All patients underwent a laparoscopic GBP with a 30-ml gastric pouch, 40-cm afferent limb, 150-cm Roux limb, and 12-mm gastrojejunostomy.
GGF repair
Patients with GGF underwent laparoscopic GGF repair. Intraoperative endoscopy confirmed the presence of the GGF. Linear cutting staplers were used to resect the body of the remnant stomach and the fistula tract and revise the gastric pouch. Repeat endoscopy and air insufflation confirmed the absence of any leak.
Oral glucose tolerance test (OGTT)
All participants underwent a 2 hour OGTT before and after GGF repair (GGF COHORT, average 4 months after GGF repair), or before and 12 months after GBP (CONTROLS). At each study visit, after an overnight fast, body weight was recorded on a precision scale, height measured, and BMI calculated. During the OGTT, blood was drawn from an IV catheter under fasting conditions and at 15, 30, 60 and 120 minutes after ingestion of 50 g of glucose (in 200 mL of non-carbonated water), for measurement of circulating concentrations of glucose, insulin, ghrelin, PYY3-36, GIP, and total GLP-1.
Assays
Glucose was measured using the glucose oxidase method (glucose analyzer; Analox Instruments USA, Lunenberg, MA). Insulin, total ghrelin, PYY3-36, and total GLP-1 were measured by radio-immuno assay (RIA) and GIP by enzyme-linked immunosorbent assay (ELISA) (Millipore, St. Charles, MO) in the Hormone and Metabolite Core laboratory of the Obesity Nutrition Research Center.
Statistical Analysis
Statistical analyses were performed with SPSS 19 (SPSS Inc., Chicago). Paired t-tests were used to assess longitudinal changes within groups and Spearman correlation was used to assess relationships between variables. Two-hour total AUC was calculated with the trapezoidal method. Data distribution was checked for normality. Data are presented as mean ± SD and the level of significance was p<0.05. Independent samples t-tests were used to compare GGF COHORT with the CONTROLS.
Results
Controls
Controls (n=22), age 45.0±9.2 years, with a pre-operative BMI of 45.7±6.5 kg/m2, lost 38.2±11.5 kg at 12.6±1.7 months post-GBP. Fasting glucose and insulin and glucose AUC0-120, decreased significantly, while fasting ghrelin, ghrelin nadir, ghrelin AUC0-120, GLP-1 AUC0-120, and PYY3-36 AUC0-120 all increased significantly 1 year after GBP (Table 1 and Figure 2).
Table 1.
Subject Characteristics, Glucose, and Hormone Levels
| Pre-GGF | Post-GGF | Pre-GBP | Post-GBP | |
|---|---|---|---|---|
| Age (Years) |
36.7±10.2 | 45.0±9.2 | ||
| BMI (kg·m−2) |
36.9±7.6 | 34.6±7.2 * | 45.7±6.5 *# | 31.3±4.1 *$ |
| Weight (kg) |
101.5±33.6 | 95.4±33.2 * | 120.5±20.0 # | 82.3±13.5 *$ |
| Fasting glucose (mmol·L−1) |
6.8±3.8 | 5.4±1.1 | 7.4±2.4 # | 4.9±0.7 $ |
| Glucose AUC0-120 (mmol·L−1·min−1) |
9.4±6.0 | 8.1±3.4 | 10.8±3.0 | 7.7±1.7 $ |
| Fasting insulin (pmol·L−1) |
105.8±40.1 | 105.3±31.2 | 210.4±106.9 *# | 70.6±32.7 *#$ |
| HOMA-IR | 4.8±3.9 | 3.7±1.6 | 9.5±5.9 *# | 2.2±1.1 #$ |
| Insulin AUC0-120 (pmol·L−1·min−1) |
396.2±157.7 | 500.1±194.2 | 479.3±274.0 | 447.9±251.6 |
| Fasting ghrelin (pg·mL−1) |
589.5±236.5 | 403.0±342.6 * | 538.7±222.3 | 758.6±513.2 $ |
| Ghrelin nadir (pg·mL−1) |
425.4±141.8 | 300.0±190.2 * | 493.9±206.1 | 590.3±367.7#$ |
| Ghrelin AUC0-120 (pg·mL −1·min−1) |
474.0±162.3 | 357.3±231.9 * | 538.5±204.6 | 646.5±391.4#$ |
| GIP AUC0-120 (pg·mL−1·min−1) |
128.7±34.4 | 135.9±44.3 | 127.6±39.8 | 143.1±57.8 |
| GLP-1AUC0-120 (pmol·L−1·min−1) |
22.8±6.5 | 25.5±6.3 | 9.5±7.1 *# | 54.6±44.0 *#$ |
| PYY3-36AUC0-120 (pmol·L−1·min−1) |
69.3±28.3 | 90.9±21.2* | 58.2±34.7 # | 106.0±35.1 *$ |
Values are mean ± SD.
Significant differences are denoted by: = p<.05 compared to Pre-GGF,
= p<.05 compared to Post-GGF,
= p<.05 compared to Pre-GBP.
n=7 for GGF, and n=22 for GBP controls
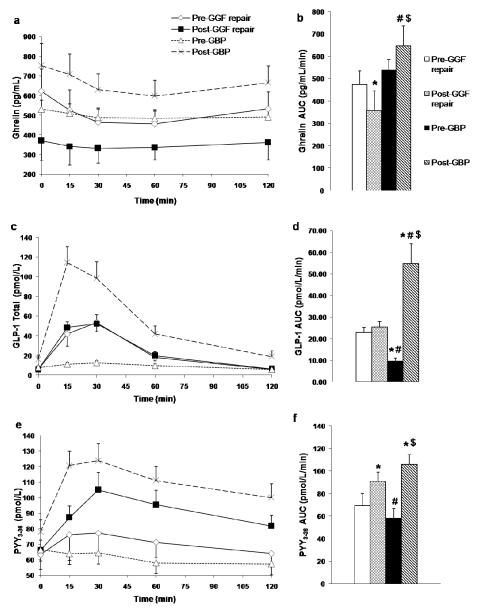
Fig. 2.
Gut Hormone Levels. Ghrelin (a,b), total GLP-1 (c,d), and PYY3-36 (e,f) levels during a 2-hr OGTT with AUC measurements. Significant differences are denoted by: * = p<.05 compared to Pre-GGF, # = p<.05 compared to Post-GGF, $ = p<.05 compared to Pre-GBP. n=7 for GGF, and n=22 for GBP controls. Data are mean ± SEM.
GGF Cohort
Prior to surgery, self-reported BMI in the GGF cohort was 51.7±14.2 kg/m2, which was not significantly different than pre-GBP BMI in the control group. Self-reported maximum weight loss after GBP was 58±31.7 kg (n=7). The main symptom that prompted the diagnosis of GGF was weight regain, reported in 4/7 subjects. Subjects regained 15.7±5.6 kg (calculated from self-reported minimum weight) 57±23 months after GBP. Characteristics of subjects are shown in Table 1. In patients post-GGF repair, weight loss resumed, with a total weight loss of 6.0±3.9 kg (p=0.006) over 4.1±4.0 months (range 2-13 months). BMI decreased significantly from 36.9±7.6 kg/m2 to 34.6±7.2 kg/m2 (p=0.008). In patients post-GGF repair, PYY3-36AUC0-120 increased significantly (p=0.010), however the enhanced PYY3-36 response to oral glucose in patients post-GGF repair did not correlate with weight loss (r=-0.143, p=0.760). Neither GLP-1 nor GIP response to oral glucose was altered in patients post-GGF repair (Table 1), compared to pre-GGF repair, and these were not correlated with weight loss.
As predicted, circulating fasting ghrelin concentrations decreased in patients post-GGF repair by approximately 30% (p=0.004), as did ghrelin AUC0-120 (p=0.006) and ghrelin nadir (p=0.016) (Table 1). Both fasting (r=0.857, p=0.014) and nadir (r=0.857, p=0.014) ghrelin concentrations strongly correlated with the amount of weight loss in patients post-GGF repair.
There was a 26% increase in the insulin response to oral glucose in patients post-GGF repair, that tended to be significant (p=.09) (Table 1). There was a non-significant decrease in fasting and glucose AUC0-120, and HOMA-IR after GGF repair. Two patients who had T2DM at the time of the diagnosis of GGF had marked reductions in fasting glucose levels (from 12.3±1.2 mmol/L to 6.8±0.9 mmol/L) and 120 min glucose (from 14.4±2.2 mmol/L to 9.9±4.2 mmol/L) after GGF repair, with a mean weight loss of 9.4±0.7 kg. Neither fasting nor AUC glucose or insulin were correlated with weight loss
Comparison of the GGF cohort with the Controls
There was no significant difference in body weight between the controls pre-GBP compared to patients pre-GGF repair, or between the controls post-GBP compared to patients post-GGF repair (Table 1). Pre-GGF repair patients differed significantly from controls both pre-GBP and post-GBP on a number of parameters. The pre-GGF repair group had significantly lower BMI, fasting insulin and HOMA-IR and significantly higher GLP-1 AUC0-120 (Table 1), and peak GLP-1 (56.6±27.1 pmol/L vs. 15.7±12.1 pmol/L, p=0.007) than the controls pre-GBP. Patients pre-GGF repair were heavier than controls post-GBP (BMI 36.9 ± 7.6 kg·m−2 vs. 31.3 ± 4.1 kg·m−2, p=0.018) and had a 58% and 35% lower GLP-1 and PYY3-36 response to oral glucose, respectively (GLP-1 AUC0-120 22.8 ± 6.5 pmol/L/min vs. 54.6 ± 44.0 pmol/L/min, p=0.003; PYY3-36 AUC0-120 69.3 ± 28.3 pmol/L/min vs. 106.0 ± 35.1 pmol/L/min, p=0.047) (Table 1). As expected post-GGF repair, ghrelin AUC0-120 (p=0.033), nadir (p=.016) and fasting (p=.059) levels were lower than post-GBP controls. Unexpectedly, the GLP-1 response to oral glucose, which was lower in the patients pre-GGF repair, compared to post-GBP controls, remained significantly lower in patients post-GGF repair (p=0.006). There was no change in magnitude of the GLP-1 peak (56.6±27.1 pmol/L vs. 54.8±22.1 pmol/L, p=0.752) in the post-GGF repair group compared to pre-GGF repair. Contrary to GLP-1 levels, PYY3-36 AUC0-120 levels post-GGF repair were no longer significantly different from the post-GBP controls (p=0.169).
As a proxy for liquid gastric emptying (GE), we measured the time to reach peak serum glucose and the time to reach peak serum GLP-1 during the 2-hr OGTT. The post-GBP controls experienced an earlier peak glucose (64.1±19.2 min vs. 38.9±15.1 min, p<0.001) and peak GLP-1 (33.4±25.7 min vs. 17.7±5.9 min, p=0.010) one year after surgery compared to pre-GBP. However, GGF repair did not significantly decrease the time to peak glucose (pre GGF vs. post-GGF, 32.1±13.5 min vs. 34.3±11.3 min, p=0.356) or the time to peak GLP-1 (30.0±15.0 min vs. 23.6±8.0 min, p=0.448), although a non-significant trend was evident in the latter. There was a significant difference in time to peak glucose between the pre-GGF repair and pre-GBP (32.1±13.5 min vs. 64.1±19.2 min, p<0.001) and no difference between the post-GGF repair and post-GBP (34.3±11.3 min vs. 38.9±15.1 min, p<0.408). There was no significant difference in time to peak GLP-1 between the GGF cohort compared to the GBP control cohort, during either the pre or post conditions.
Discussion
We hypothesized that 1) GGF repair would lead to weight loss; 2) GLP-1, GIP and PYY3-36 response to oral glucose would be blunted in patients pre-GGF repair and would increase after GGF repair to levels comparable to the post-GBP group; and 3) circulating ghrelin concentrations would decrease after GGF repair and be significantly lower than the post-GBP group. These expected gut peptides changes in the post-GGF repair group would be consistent with the foregut hypothesis as well as expected changes after a gastrectomy. Our data show that approximately 4 months after GGF repair, weight loss resumed and yielded favorable changes in satiety and orexigenic gut hormones. After GGF repair, subjects lost an average of 6 kg, resulting in weight and BMI values that were not significantly different from the post-GBP control group. There was a very slight non-significant increase in the anorectic hormone GLP-1 after GGF repair. However, GLP-1 levels in both the pre- and post-GGF repair group were significantly lower compared to the post-GBP controls and higher compared to the pre-GBP controls. Oppositely, PYY3-36, another anorectic satiety hormone, was significantly increased after GGF repair and no longer significantly lower than the post-GBP control group. The increase in PYY3-36 after GGF repair lends support to the theory that GGF could short-circuit the hormonal effect of GBP, and that GGF repair restores some features of the expected post-GBP physiology. This hypothesis would purport an increase in GLP-1 levels after surgery as well, however no significant increase was observed. This is a puzzling observation, particularly since PYY3-36 levels were elevated after GGF repair. PYY3-36, like GLP-1, is secreted from L-cells in the distal ileum, and this increased secretion in gut hormones from L-cells may be due to increased GE rates after GBP [11,12]. However, evidence suggests that in the GGF group, GE rates were already reduced to levels of the post-GBP controls, and GGF repair did not further accelerate GE. It is also possible that the lack of significance is due to the small number of subjects, as well as the variability observed with GLP-1 response over time [13]. Furthermore, we measured total, not active GLP-1, and although these are correlated, we cannot rule out that perhaps there is increased active GLP-1 [14].
After GGF repair, we also observed a reduction in fasting, nadir and postprandial levels of the orexigenic hormone ghrelin; in fact, ghrelin was significantly lower in the post-GGF repair group compared to the post-GBP controls. This was consistent with our hypothesis, as GGF repair required the surgical removal of the gastric remnant, and thus the removal of the majority of ghrelin-producing cells [15]. This could potentially provide an additional weight loss effect in addition to that from GBP surgery. This is similar to what has been previously reported in subjects undergoing GBP with gastric fundus resection. Ghrelin levels after this procedure were reduced up to 1 year after surgery, while subjects undergoing GBP alone had a transient decrease in ghrelin levels up to 3 months, and increase in levels by 1 year [16]. In our current study, ghrelin levels were significantly increased 1 year after GBP (pre-GBP vs. post-GBP), in proportion with weight loss, which is in agreement with what has been previously shown by our group and others [9,17,18]. Hence, the sustained weight loss observed in the GBP controls suggests that the changes in GLP-1 and PYY3-36 observed in the post-GBP group may play a role in promoting weight loss, but the change in ghrelin may not. Increased GLP-1 and PYY3-36 after GBP surgery is in agreement with other studies [19-21] . It is tempting to speculate that the increased levels of the anorectic hormone PYY3-36 and decrease in the orexigenic hormone ghrelin may, in part, play a role in the weight loss observed in the post-GGF repair group; however this is unclear as our data is observational.
There was some limited evidence for an improvement in glucose metabolism after GGF repair. There was a non-significant improvement in fasting glucose, glucose AUC, and HOMA-IR, and a non-significant increase in fasting insulin and insulin AUC. This trending increase in insulin AUC was observed without a concomitant increase in the incretin GLP-1 after GGF repair. The lack of significance with respect to other glucose parameters is likely due to the small sample size with a mixed diabetes status. These improvements in glucose metabolism are likely due to the reduction in body weight observed after GGF, rather than the modest changes in incretins GLP-1 and GIP. Although PYY3-36 has been shown to improve insulin sensitivity in mice independently of weight [22], the results in primates and humans are mixed [23,24] and hence the increased PYY3-36 levels are likely not mediating the tendency for improved glucose metabolism in this study.
We [20] and others [25,26] have previously shown that an increase in GLP-1 after GBP is implicated in the improvement in glucose metabolism after surgery. In this study, only two individuals had T2DM prior to GGF repair, and although diabetes improved in these individuals, we cannot draw any definitive conclusions with such small numbers. Future studies in subjects before and after GGF repair could more carefully assess how rapidly diabetes improves after repair by examining glucose metabolism shortly after GGF repair, prior to any measureable amount of weight loss. Similarly, long term studies in post-GBP patients with GGF complications could help elucidate the mechanisms responsible for weight regain and diabetes relapse after GBP surgery.
Although numerous studies have shown enhanced incretin and PYY release after GBP [19-21,25-29], the mechanisms by which this occurs are not fully understood. Two potential theories referred to as the foregut hypothesis and the hindgut hypothesis, were initially described [5,6]. The foregut hypothesis suggests that favorable changes in glucose metabolism after bariatric surgery can be attributed to exclusion of the proximal small bowel, including the duodenum and part of the jejunum. This is supported by several animal and human studies which exclude nutrients from the proximal small bowel either via intestinal bypass, endoluminal sleeves or GBP. It has been shown that gastrojejunal bypass in the Goto-Kakizaki (GK) rat, a non-obese animal model with type 2 diabetes, improved glucose tolerance independently of weight loss [5,30]. Duodenal-jejunal exclusion via an endoluminal sleeve in rats also improved glucose tolerance and increased GLP-1 levels independent of weight loss [8]. In GBP patients, it was shown that a glucose load administered orally induced greater plasma insulin and gut hormone (GLP-1 and PYY) responses compared with glucose loading via a gastrostomy tube [31], and studies with an endoluminal sleeve in humans have reported improved glucose metabolism [32,33]. On the other hand, vertical sleeve gastrectomy (VSG), a purely restrictive procedure that removes the majority of the stomach but does not bypass any of the small intestine, has been shown to improve glucose metabolism independent of weight loss in both rodents [34] and humans [35,36], and increase GLP-1 to post-GBP levels [34-36]. Thus, data from VSG studies invalidate the foregut hypothesis as there is no proximal small bowel exclusion with this procedure. This suggests that other mechanisms may be responsible for the improvement in glycemic control and the rise in gut peptides after surgery.
The hindgut hypothesis suggests that rapid nutrient delivery to the distal ileum leads to increased GLP-1 and PYY, and beneficial effects on glucose metabolism, independent of weight loss [7,11,37,38]. In fact, ileal transposition to the proximal intestine has been shown to be equivalent to duodenal-jejunal bypass in improving glucose tolerance and increasing GLP-1 levels [7,39]. Studies highlighting GE, which is accelerated after liquid consumption in GBP patients [12,19,40], also support the hindgut hypothesis. In a case study, McLaughlin et al showed that meal administration via a gastrostomy tube directly into the gastric remnant ameliorated hypoglycemia as well as hypersecretion of GLP-1 and insulin in a post-GBP patient with neuroglycopenia [41]. Hansen et al observed that meal administration to post-GBP patients either orally (bypassing the gastric remnant and proximal intestine) or via a gastrostomy tube into the gastric remnant yielded similar effects on glucose and GLP-1, suggesting that accelerated nutrient delivery plays a role in enhanced incretin response and improved glucose metabolism [42]. These pioneering studies in humans by McLaughlin and Hansen [41,42] are one of the first to directly investigate the impact of the route of nutrient delivery on gut hormone secretion in post-GBP subjects. Future studies using direct delivery of nutrients to different parts of the intestine are required to determine the impact of the route of nutrient delivery on gut hormone levels and weight and glucose parameters
Although this study utilizes a unique model to study the potential effects of a dual route of nutrient delivery on weight and glucose-related parameters as well as gut hormone levels, there are some limitations. Firstly, we do not have pre-GBP glucose or hormone levels in the GGF cohort, to ascertain that these cohorts were optimally matched prior to GBP surgery. However, we do have two major characteristics pre-GBP in the GGF cohort, weight and age, and these were not significantly different between the two cohorts prior to GBP surgery. Secondly, we cannot assume that GGF size and anatomy was consistent between subjects. While it would be interesting to correlate fistula size and hormone levels prior to GGF repair, we do not have this detailed information for all subjects. In a related example, although stoma diameter after GBP does not appear to influence the rate of GE [43], stoma diameter has been shown to be inversely correlated with weight loss after GBP [44]. We suspect that a larger GGF would be hypothesized to secrete more nutrients into the remnant stomach and thus dampen the expected increase in PYY and GLP-1 expected after GBP, which could contribute to weight re-gain. Thus, although these observations are unique and timely, they should we interpreted with recognition of their limitations.
GGF is a serious but rare complication of GBP surgery, occurring in less than 2% of subjects, with a greater incidence after undivided open GBP versus divided open or closed surgery [10,45]. GGF usually requires surgical repair with removal of the fistula and surrounding necrotic tissue, potentially resulting in a complete remnant gastrectomy and a decreased gastric pouch size [4,46,47]. This rare clinical complication may be accompanied with weight regain and/or diabetes relapse [10]. GGF occurrence is rare at our institution (estimated 1.3%, data not shown), and thus our study has a limited sample size and follow-up after GGF repair. However this model complication provides a unique, non-invasive opportunity to study the effects of a dual route versus single route of nutrient delivery on gut peptides, glucose and energy metabolism and has the potential to provide insight into the mechanisms of diabetes relapse and weight regain.
Acknowledgements
We thank our participants, the technicians from the NYONRC Hormonal core laboratory, Yim Dam and Ping Zhou, Dr. Julio Teixeira for referring patients, and the GCRC-CTSA staff.
The study was supported by grants from the St Luke’s-Roosevelt Hospital Pilot Research Program, the American Diabetes Association (CR-7-05 CR-18), the National Institutes of Health (R01-DK067561, P30-DK026687, P30-DK063068), and Columbia University’s CTSA grant No. UL1 RR024156 from NCRR/NIH.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Ciaran O’Brien, Gary Wang, James McGinty, Keesandra Agenor, Roxanne Dutia, Antonia Colarusso, Koji Park, Ninan Koshy, and Blandine Laferrère declare that there are no conflicts of interest.
Author Contributions
Data collection: CO, KA, TC, GW, BL.
Data and Statistical analysis: CO, GW, BL.
Manuscript writing and/or editing: CO, KA, GW, RD, JM, NK, KP, BL.
Study Design: BL
Reference List
- 1.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–256. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 2.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christou NV, Look D, MacLean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244(5):734–740. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cucchi SG, Pories WJ, MacDonald KG, et al. Gastrogastric fistulas. A complication of divided gastric bypass surgery. Ann Surg. 1995;221(4):387–391. doi: 10.1097/00000658-199504000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239(1):1–11. doi: 10.1097/01.sla.0000102989.54824.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strader AD. Ileal transposition provides insight into the effectiveness of gastric bypass surgery. Physiol Behav. 2006;88(3):277–282. doi: 10.1016/j.physbeh.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 7.Kindel TL, Yoder SM, Seeley RJ, et al. Duodenal-jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J Gastrointest Surg. 2009;13(10):1762–1772. doi: 10.1007/s11605-009-0912-9. [DOI] [PubMed] [Google Scholar]
- 8.Munoz R, Carmody JS, Stylopoulos N, et al. Isolated Duodenal Exclusion Increases Energy Expenditure and Improves Glucose Homeostasis in Diet-induced Obese Rats. Am J Physiol Regul Integr Comp Physiol. 2012;303(10):R985–93. doi: 10.1152/ajpregu.00262.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bose M, Machineni S, Olivan B, et al. Superior appetite hormone profile after equivalent weight loss by gastric bypass compared to gastric banding. Obesity (Silver Spring) 2010;18(6):1085–1091. doi: 10.1038/oby.2009.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrodeguas L, Szomstein S, Soto F, et al. Management of gastrogastric fistulas after divided Roux-en-Y gastric bypass surgery for morbid obesity: analysis of 1,292 consecutive patients and review of literature. Surg Obes Relat Dis. 2005;1(5):467–474. doi: 10.1016/j.soard.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Strader AD, Vahl TP, Jandacek RJ, et al. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288(2):E447–E453. doi: 10.1152/ajpendo.00153.2004. [DOI] [PubMed] [Google Scholar]
- 12.Wang G, Agenor K, Pizot J, et al. Accelerated gastric emptying but no carbohydrate malabsorption 1 year after gastric bypass surgery (GBP) Obes Surg. 2012;22(8):1263–1267. doi: 10.1007/s11695-012-0656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van der Schueren BJ, Homel P, Alam M, et al. Magnitude and variability of the glucagon-like peptide-1 response in patients with type 2 diabetes up to 2 years following gastric bypass surgery. Diabetes Care. 2012;35(1):42–46. doi: 10.2337/dc11-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heijboer AC, Frans A, Lomecky M, et al. Analysis of glucagon-like peptide 1; what to measure? Clin Chim Acta. 2011;412(13-14):1191–1194. doi: 10.1016/j.cca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Ariyasu H, Takaya K, Tagami T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86(10):4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 16.Chronaiou A, Tsoli M, Kehagias I, et al. Lower Ghrelin Levels and Exaggerated Postprandial Peptide-YY, Glucagon-Like Peptide-1, and Insulin Responses, After Gastric Fundus Resection, in Patients Undergoing Roux-en-Y Gastric Bypass: A Randomized Clinical Trial. Obes Surg. 2012;22(11):1761–1770. doi: 10.1007/s11695-012-0738-5. [DOI] [PubMed] [Google Scholar]
- 17.Faraj M, Havel PJ, Phelis S, et al. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88(4):1594–1602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 18.Sundbom M, Holdstock C, Engstrom BE, et al. Early changes in ghrelin following Rouxen-Y gastric bypass: influence of vagal nerve functionality? Obes Surg. 2007;17(3):304–310. doi: 10.1007/s11695-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 19.Morinigo R, Moize V, Musri M, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91(5):1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 20.Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30(7):1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olivan B, Teixeira J, Bose M, et al. Effect of weight loss by diet or gastric bypass surgery on peptide YY3-36 levels. Ann Surg. 2009;249(6):948–953. doi: 10.1097/SLA.0b013e3181a6cdb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Hoek AM, Heijboer AC, Voshol PJ, et al. Chronic PYY3-36 treatment promotes fat oxidation and ameliorates insulin resistance in C57BL6 mice. Am J Physiol Endocrinol Metab. 2007;292(1):E238–E245. doi: 10.1152/ajpendo.00239.2006. [DOI] [PubMed] [Google Scholar]
- 23.Sloth B, Davidsen L, Holst JJ, et al. Effect of subcutaneous injections of PYY1-36 and PYY3-36 on appetite, ad libitum energy intake, and plasma free fatty acid concentration in obese males. Am J Physiol Endocrinol Metab. 2007;293(2):E604–E609. doi: 10.1152/ajpendo.00153.2007. [DOI] [PubMed] [Google Scholar]
- 24.Koegler FH, Enriori PJ, Billes SK, et al. Peptide YY(3-36) inhibits morning, but not evening, food intake and decreases body weight in rhesus macaques. Diabetes. 2005;54(11):3198–3204. doi: 10.2337/diabetes.54.11.3198. [DOI] [PubMed] [Google Scholar]
- 25.Dar MS, Chapman WH, III, Pender JR, et al. GLP-1 response to a mixed meal: what happens 10 years after Roux-en-Y gastric bypass (RYGB)? Obes Surg. 2012;22(7):1077–1083. doi: 10.1007/s11695-012-0624-1. [DOI] [PubMed] [Google Scholar]
- 26.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011;60(9):2308–2314. doi: 10.2337/db11-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morinigo R, Lacy AM, Casamitjana R, et al. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16(12):1594–1601. doi: 10.1381/096089206779319338. [DOI] [PubMed] [Google Scholar]
- 28.Borg CM, le Roux CW, Ghatei MA, et al. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93(2):210–215. doi: 10.1002/bjs.5227. [DOI] [PubMed] [Google Scholar]
- 29.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 30.Pacheco D, de Luis DA, Romero A, et al. The effects of duodenal-jejunal exclusion on hormonal regulation of glucose metabolism in Goto-Kakizaki rats. Am J Surg. 2007;194(2):221–224. doi: 10.1016/j.amjsurg.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Pournaras DJ, Aasheim ET, Bueter M, et al. Effect of bypassing the proximal gut on gut hormones involved with glycemic control and weight loss. Surg Obes Relat Dis. 2012;8(4):371–374. doi: 10.1016/j.soard.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Grunert L, Galvao Neto MP, Alamo M, et al. First human experience with endoscopically delivered and retrieved duodenal-jejunal bypass sleeve. Surg Obes Relat Dis. 2008;4(1):55–59. doi: 10.1016/j.soard.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Escalona A, Pimentel F, Sharp A, et al. Weight loss and metabolic improvement in morbidly obese subjects implanted for 1 year with an endoscopic duodenal-jejunal bypass liner. Ann Surg. 2012;255(6):1080–1085. doi: 10.1097/SLA.0b013e31825498c4. [DOI] [PubMed] [Google Scholar]
- 34.Chambers AP, Jessen L, Ryan KK, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141(3):950–958. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22(5):740–748. doi: 10.1007/s11695-012-0622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramon JM, Salvans S, Crous X, et al. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg. 2012;16(6):1116–1122. doi: 10.1007/s11605-012-1855-0. [DOI] [PubMed] [Google Scholar]
- 37.Cummings BP, Strader AD, Stanhope KL, et al. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology. 2010;138(7):2437–46. 2446. doi: 10.1053/j.gastro.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patriti A, Facchiano E, Annetti C, et al. Early improvement of glucose tolerance after ileal transposition in a non-obese type 2 diabetes rat model. Obes Surg. 2005;15(9):1258–1264. doi: 10.1381/096089205774512573. [DOI] [PubMed] [Google Scholar]
- 39.Wang TT, Hu SY, Gao HD, et al. Ileal transposition controls diabetes as well as modified duodenal jejunal bypass with better lipid lowering in a nonobese rat model of type II diabetes by increasing GLP-1. Ann Surg. 2008;247(6):968–975. doi: 10.1097/SLA.0b013e318172504d. [DOI] [PubMed] [Google Scholar]
- 40.Horowitz M, Collins PJ, Harding PE, et al. Gastric emptying after gastric bypass. Int J Obes. 1986;10(2):117–121. [PubMed] [Google Scholar]
- 41.McLaughlin T, Peck M, Holst J, et al. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab. 2010;95(4):1851–1855. doi: 10.1210/jc.2009-1628. [DOI] [PubMed] [Google Scholar]
- 42.Hansen EN, Tamboli RA, Isbell JM, et al. Role of the foregut in the early improvement in glucose tolerance and insulin sensitivity following Roux-en-Y gastric bypass surgery. Am J Physiol Gastrointest Liver Physiol. 2011;300(5):G795–G802. doi: 10.1152/ajpgi.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horowitz M, Cook DJ, Collins PJ, et al. Measurement of gastric emptying after gastric bypass surgery using radionuclides. Br J Surg. 1982;69(11):655–657. doi: 10.1002/bjs.1800691108. [DOI] [PubMed] [Google Scholar]
- 44.Heneghan HM, Yimcharoen P, Brethauer SA, et al. Influence of pouch and stoma size on weight loss after gastric bypass. Surg Obes Relat Dis. 2012;8(4):408–415. doi: 10.1016/j.soard.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Yao DC, Stellato TA, Schuster MM, et al. Gastrogastric fistula following Roux-en-Y bypass is attributed to both surgical technique and experience. Am J Surg. 2010;199(3):382–385. doi: 10.1016/j.amjsurg.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Filho AJ, Kondo W, Nassif LS, et al. Gastrogastric fistula: a possible complication of Roux-en-Y gastric bypass. JSLS. 2006;10(3):326–331. [PMC free article] [PubMed] [Google Scholar]
- 47.Bhardwaj A, Cooney RN, Wehrman A, et al. Endoscopic repair of small symptomatic gastrogastric fistulas after gastric bypass surgery: a single center experience. Obes Surg. 2010;20(8):1090–1095. doi: 10.1007/s11695-010-0180-5. [DOI] [PubMed] [Google Scholar]