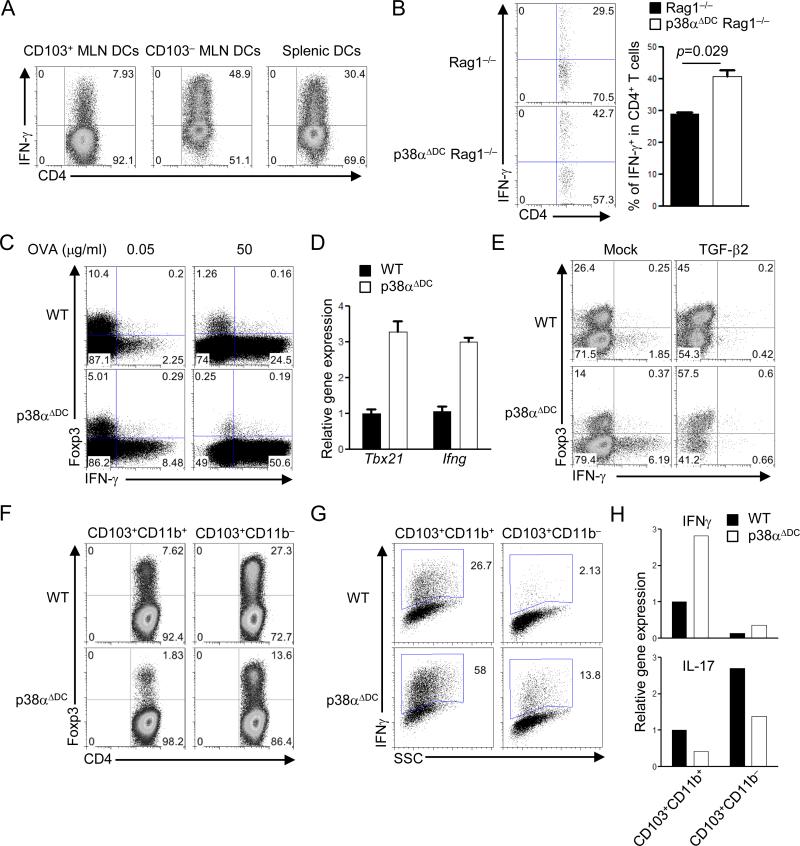
Figure 5. DC-derived p38α signaling regulates reciprocal iTreg and Th1 differentiation in a TGF-β2-dependent manner.
(A) Expression of IFN-γ in OT-II T cells activated with CD103+ and CD103– MLN DCs and splenic DCs, in the presence of OVA (0.05 μg/ml), for 5 days, followed by intracellular cytokine staining. (B) T cells were transferred into Rag1–/– and p38αΔDC Rag1–/– mice, and expression of IFN-γ from donor cells in MLNs was analyzed at day 7. Right, the proportions of IFN-γ+ population among donor CD4+ T cells. (C) Expression of Foxp3 and IFN-γ in OT-II T cells stimulated with WT or p38α-deficient CD103+ DCs, in the presence of the indicated doses of OVA, for 5 days. (D) Gene expression in T cells from (C). (E) Expression of Foxp3 and IFN-γ in OT-II T cells stimulated with WT or p38α-deficient CD103+ DCs, in the presence of OVA (0.05 μg/ml), with or without TGF-β2 for 5 days. (F–G) Expression of Foxp3 (F) and IFN-γ (G) in OT-II T cells stimulated with WT or p38α-deficient CD103+CD11b+ or CD103+CD11b– MLN DCs, in the presence of OVA (0.05 μg/ml), for 5 days. (H) RNA analysis of IFNγ and IL-17 expression from (F–G). Data are representative of 2 (n=5 mice per group) independent experiments. Error bars indicate SEM.