Abstract
Prevention has a paramount role in reducing the incidence of corrosive ingestion especially in children, yet this goal is far from being reached in developing countries, where such injuries are largely unreported and their true prevalence simply cannot be extrapolated from random articles or personal experience. The specific pathophysiologic mechanisms are becoming better understood and may have a role in the future management and prevention of long-term consequences, such as esophageal strictures. Whereas the mainstay of diagnosis is considered upper gastrointestinal endoscopy, computed tomography and ultrasound are gaining a more significant role, especially in addressing the need for emergency surgery, whose morbidity and mortality remains high even in the best hands. The need to perform emergency surgery has a persistent long-term negative impact both on survival and functional outcome. Medical or endoscopic prevention of stricture is debatable, yet esophageal stents, absorbable or not, show promising data. Dilatation is the first therapeutic option for strictures and bougies should be considered especially for long, multiple and tortuous narrowing. It is crucial to avoid malnutrition, especially in developing countries where management strategies are influenced by malnutrition and poor clinical conditions. Late reconstructive surgery, mainly using colon transposition, offers the best results in referral centers, either in children or adults, but such a difficult surgical procedure is often unavailable in developing countries. Possible late development of esophageal cancer, though probably overemphasized, entails careful and long-term endoscopic screening.
Keywords: Caustic ingestion, Corrosive stricture, Developing countries, Surgical management, Endoscopic management
Core tip: The incidence of corrosive ingestion is high and largely unreported in developing countries, where prevention is lacking. Computed tomography and endoscopic ultrasound are gaining a more meaningful role in addressing the need for emergency surgery. The need to perform emergency surgery has a persistent long-term negative impact both on survival and functional outcome. Prevention of stricture is still a debatable issue, yet esophageal stents may offer promising outcomes. It is crucial to avoid malnutrition, especially in developing countries where management strategies are conditioned by poor clinical conditions. Late reconstructive surgery is often unavailable in developing countries.
INTRODUCTION
Ingestion of corrosive substances remain an important public health issue in Western countries despite education and regulatory efforts to reduce its occurrence. These injuries are still increasing in developing countries[1,2], related to the social, economic, and educational variables and mainly to a lack of prevention[3,4]. The problem is largely unreported in these settings and its true prevalence simply cannot be extrapolated from the scarce papers or personal experience. Data available are heavily skewed towards well-resourced centers and do not mirror the full reality of the condition. Moreover, in industrialized and developing countries, the therapeutic approach and management strategies appear to be different, likely because of technology and endoscopic expertise.
Two independent MEDLINE and EMBASE searches from 1990-2012 were performed to identify relevant articles. The following medical subject headings terms were used in the searches: caustic ingestion, caustic lesions, corrosive injuries, esophagus, esophageal dilatation. Bibliographies of retrieved studies were reviewed and general medical and major gastroenterology journals manually searched over the previous 5 years.
EPIDEMIOLOGY AND PATHOPHYSIOLOGY
Worldwide, children represent 80% of the ingestion injury population globally[5], primarily due to accidental ingestion[6]. In contrast, ingestion in adults is more often suicidal in intent, and is frequently life-threatening.
Traditionally, ingested corrosive substances are either alkalis or acids (Table 1). Alkaline material accounts for most caustic ingestions in Western countries whereas injuries from acid are more common in some developing countries, like India, where hydrochloric acid and sulfuric acid are easily accessible[7]. Acids and alkalis produce different types of tissue damage. Acids cause coagulation necrosis, with eschar formation that may limit substance penetration and injury depth[8]. Conversely, alkalis combine with tissue proteins and cause liquefactive necrosis and saponification, and penetrate deeper into tissues, helped by a higher viscosity and a longer contact time through the esophagus. Additionally, alkali absorption leads to thrombosis in blood vessels, impeding blood flow to already damaged tissue[9]. Injury occurs quickly, depending on the agent’s concentration and time of exposure (Figure 1)[10], with a 30% solution of sodium hydroxide being able to produce full thickness injury in 1 s[11]. Accordingly, alkali ingestion may lead to more serious injury and complications, but this distinction is probably not clinically relevant in the setting of strong acid or base ingestion, both being able to penetrate tissues rapidly, potentially leading to full-thickness damage of the esophageal/gastric wall. The conventional acceptance that acids preferentially damage the stomach, due to the protective esophageal eschar, has recently been questioned, with observation of extensive esophageal damage and perforations after acid ingestion[12]. Likewise, compared with alkali, ingestion of a strong acid may be associated with a higher incidence of systemic complications, such as renal failure, liver dysfunction, disseminated intravascular coagulation and hemolysis[13].
Table 1.
Most commonly ingested caustic substances
| Caustic substance | Type | Commercially available form |
| Acids | Sulfuric | Batteries |
| Industrial cleaning agents | ||
| Metal plating | ||
| Oxalic | Paint thinners, strippers | |
| Metal cleaners | ||
| Hydrochloric | Solvents | |
| Metal cleaners | ||
| Toilet and drain cleaners | ||
| Antirust compounds | ||
| Phosphoric | Toilet cleaners | |
| Alkali | Sodium hydroxide | Drain cleaners |
| Home soap manufacturing | ||
| Potassium hydroxide | Oven cleaners | |
| Washing powders | ||
| Sodium carbonate | Soap manufacturing | |
| Fruit drying on farms | ||
| Ammonia | Commercial ammonia | Household cleaners |
| Ammonium hydroxide | Household cleaners | |
| Detergents, bleach | Sodium hypochlorite | Household bleach, cleaners |
| Sodium polyphosphate | Industrial detergents | |
| Condy’s crystals | Potassium permanganate | Disinfectants, hair dyes |
Figure 1.
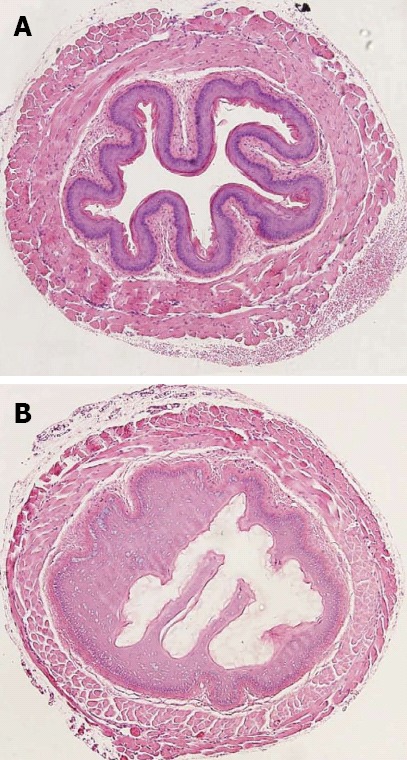
Murine esophagus exposed for 10 min to control (A) and 10% NaOH (B). Reproduced from Osman et al[10].
Esophageal injury begins within minutes and may persist for hours. Initially, tissue injury is marked by eosinophilic necrosis with swelling and hemorrhagic congestion[9]. Experimental findings suggest that arteriolar and venular thrombosis with consequent ischemia may be more important than inflammation in the pathogenesis of acute corrosive injury[10]. Four to 7 d after ingestion, mucosal sloughing and bacterial invasion are the main findings. At this time granulation tissue appears, and ulcers become covered by fibrin. Perforation may occur during this period if ulceration exceeds the muscle plane. Fibroblasts appear at the injury site around day 4, and around day 5, an “esophageal mold’’ is formed, consisting of dead cells and secretions. Esophageal repair usually begins on the 10th day after ingestion, whereas esophageal ulcerations begin to epithelialize approximately 1 mo after exposure. The tensile strength of the healing tissue is low during the first 3 wk since collagen deposition may not begin until the second week. Hence, endoscopy (and of course dilatation) is preferably avoided 5-15 d after ingestion[14]. Scar retraction begins by the third week and may continue for several months, resulting in stricture formation and shortening of the involved segment of the gastrointestinal tract. Additionally, lower esophageal sphincter pressure becomes impaired, leading to increased gastroesophageal reflux (GER), which in turn accelerates stricture formation[15]. GER is indeed a likely significant factor in persistent strictures not responding to sequential esophageal dilatations. Esophageal motility studies report low amplitude and nonperistaltic contractions, with a significantly higher exposure to pH below 4, compared with control groups[16]. Therefore, all caustic esophageal burn patients should be screened for GER periodically, and GER should be controlled aggressively.
Reactive oxygen species generation with subsequent lipid peroxidation may contribute either to the initial esophageal injury, or to the subsequent stricture formation. Malondialdehyde, an end-product of lipid peroxidation, was found at significantly higher levels than normal in esophageal tissue exposed to sodium hydroxide, signifying the presence of reactive oxygen species at 24 h post exposure. These concentrations remained high for 72 h after exposure compared with no injured controls. Furthermore, significantly lower glutathione concentrations, a known endogenous free-radical scavenger, were found in the same tissues compared with controls, further supporting the presence of reactive oxygen species and free-radical damage[17].
CLINICAL PRESENTATION
Clinical features depend on the type of the substance, amount, physical form and time of presentation (early or delayed). Crystals or solid particles may adhere to the mucosa of the mouth, making them difficult to swallow and thereby diminishing the injury produced to the esophagus, but potentially increasing the damage to the upper airway and pharynx. Conversely, liquids are easily swallowed and are most likely to damage the esophagus and stomach, the extent of injury correlating directly with mortality and late sequelae[18,19]. Patients with oropharyngeal burns do not have significant damage to the esophagus in up to 70%, hence their presence is not a reliable index of esophageal damage[20]. Hoarseness and stridor suggest laryngeal or epiglottic involvement; dysphagia and odynophagia imply esophageal damage while epigastric pain and bleeding are more common in stomach involvement. The absence of pain does not preclude significant gastrointestinal damage. Later changes, such as appearance or worsening of abdominal or chest pain, should be carefully monitored and promptly investigated, since esophageal or gastric perforations can occur at any time during the first 2 wk after ingestion[5].
The relationship between symptoms and severity of injury is uncertain[21]. Stridor and drooling were considered 100% specific for significant esophageal injury[22,23], but no single symptom or symptom cluster can predict the degree of esophageal damage[20,24,25].
The incidence of coexistent gastric injury in the literature ranges from 20.0% to as high as 62.5%[26,27], extending from simple hyperemia/erosions to diffuse transmural necrosis. Delayed gastric emptying with consequent accumulation of food in the stomach (likely due to the contraction of the antropyloric region) may affect the severity of injuries. The most common presentation of an acute corrosive gastric burn is abdominal pain, vomiting, and hematemesis. Rarely, a full thickness burn can cause an immediate gastric perforation, which tends to present a few days after ingestion. Gastric perforation, early or delayed, carries a significant mortality[28], and is more rarely reported in children. Clinical examination and a careful follow-up with a computed tomography (CT) scan are likely more useful than endoscopy in assessing threatened or existing perforation[29]. Bleeding following corrosive ingestion is usually self-limiting: though massive hemorrhage from the stomach or duodenum has been reported a short time after corrosive ingestion[30], severe bleeding typically occurs at 2 wk after ingestion[29].
Respiratory complications from caustic ingestion may result in laryngeal injury and upper airway edema, which ultimately may require tracheotomy[31] and is usually coupled with extensive esophageal damage. Laryngeal injuries were diagnosed by flexible fiberoptic or rigid laryngoscopy in 38% of patients after caustic ingestion, but only few (8%) required immediate intubation and mechanical ventilation for respiratory distress on admission[11]. This low rate of lower airway and pulmonary complications suggests that the protective pharyngeal-glottic mechanism is highly efficient in preventing the caustic substance to reach the lower airway.
EVALUATION AND ASSESSMENT
Laboratory studies
Correlation between laboratory values and the severity/outcome of injury is poor. A high white blood cell count (> 20000 cells/mm3), elevated serum C-reactive protein, age and the presence of an esophageal ulcer have been considered predictors of mortality in adults[32]; an arterial pH less than 7.22 or a base excess lower than -12 have been considered indication of severe esophageal injury and of emergency surgery[33]. Essentially, laboratory studies are more useful in monitoring and guiding patient management than in predicting morbidity or mortality[34].
Traditional radiology
Shortly after ingestion, a plain chest radiograph may reveal air in the mediastinum suggesting esophageal perforation, as well as free air under the diaphragm, indicating gastric perforation. If it is felt necessary to confirm a clinically suspected perforation, a water-soluble agent, such as Hypaque™ or Gastrografin™, and less irritant than barium sulphate, should probably be used, though both can be equally irritant[35]. Conversely, barium sulfate should be the preferred contrast agent in late barium swallowing, providing greater radiographic details than water-soluble contrast agents[22].
Ultrasounds
Evaluation of esophageal wall caustic damage by endoscopic ultrasound (EUS) using a miniprobe seems safe, though prolongs examination time without showing any difference with endoscopy in predicting early complications[36]. The destruction of the muscular layers of the esophagus observed at EUS seems a reliable sign of future stricture formation[37]; furthermore, ultrasound examination with a radial probe may predict the response to dilatation, which usually requires more sessions when the muscolaris propria is involved at EUS, as in Figure 2[38]. In spite of these encouraging reports, the role of US examination in caustic injuries is still under evaluation.
Figure 2.
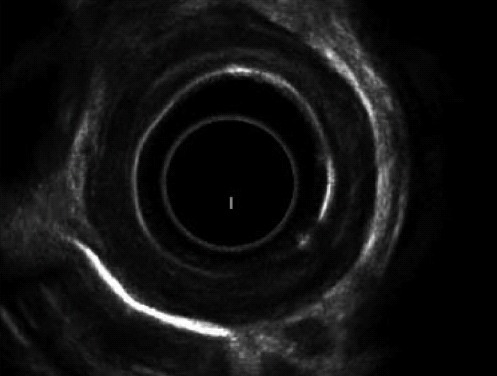
Endoscopic ultrasound showing involvement of the muscularis propria of esophageal wall. Reproduced from Kamijo et al[37].
CT scan
A CT scan likely offers a more detailed evaluation than early endoscopy about the transmural damage of esophageal and gastric walls and the extent of necrosis[39]. It is more valuable than endoscopy in assessing threatened or established stomach perforation[29], and a CT grading system (Table 2 and Figure 3) has been proposed to predict esophageal stricture[40,41]. With the advantage of not being invasive, CT scan has a promising role in the early evaluation of caustic injury damage.
Table 2.
Computed tomography grading system for caustic lesions
| Grade | Features |
| Grade 1 | No definite swelling of esophageal wall |
| Grade 2 | Edematous wall thickening without periesophageal soft tissue involvement |
| Grade 3 | Edematous wall thickening with periesophageal soft tissue infiltration plus well-demarcated tissue interface |
| Grade 4 | Edematous wall thickening with periesophageal soft tissue infiltration plus blurring of tissue interface or localized fluid collection around the esophagus or descending aorta |
Reproduced from Ryu et al[40].
Figure 3.
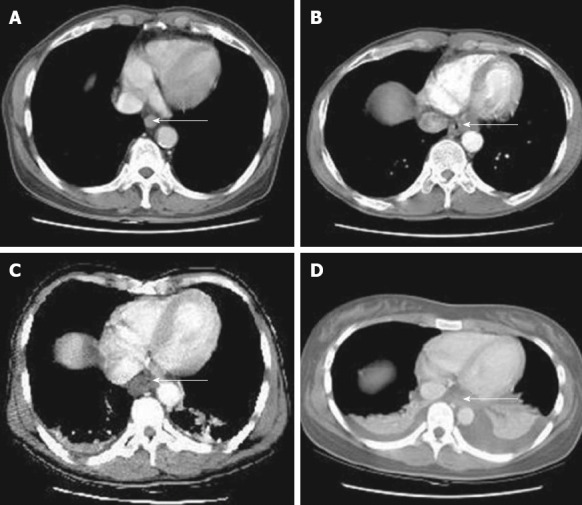
Computed tomography grading of esophageal caustic injuries. A: Grade 1; B: Grade 2; C: Grade 3; D: Grade 4. Reproduced from Ryu et al[40]. Arrows show the esophageal wall.
Endoscopy
Esophagogastroduodenoscopy is considered crucial and usually recommended in the first 12-48 h after caustic ingestion, though it is safe and reliable up to 96 h after the injury[13,42]; gentle insufflation and great caution are mandatory during the procedure. Endoscopy and even dilatation have been performed without consequences from 5 to 15 d after corrosive ingestion[43], though potentially hazardous due to tissue softening and friability during the healing period. Adequate sedation (general anesthesia in children) is compulsory, yet endotracheal intubation is strictly required only for patients in respiratory distress. The constraint to stop the endoscope in the presence of a circumferential second or third degree esophageal burn is not mandatory[44,45].
When lip and oropharyngeal injuries are the main clinical findings, esophageal or gastric injuries are generally no greater than grade 1[46]. Although severe esophageal injuries have been reported in 12.0%[47] and 19.3%[48] of asymptomatic children, significant lesions at endoscopy are not usually observed when symptoms are absent after unintentional ingestion of less aggressive substances[24,49], thus making routine post-ingestion endoscopy questionable in this group of patients. All adult patients must undergo endoscopy after suicidal ingestion, because of the larger amount of more corrosive agents swallowed compared with unintentional injuries, where early esophagoscopy has been questioned[50]. Ultimately, though endoscopy is considered by most a cornerstone in the diagnosis of corrosive ingestions, which patients would clearly benefit from it is still debated. Considering that 10%-30% of caustic ingestions globally do not show any upper gastrointestinal injury[22,51], the indication for early endoscopy should be made on a case-by-case basis, with consideration of symptoms, otorhinolaryngeal injuries, and the amount and nature of the ingested substance.
Contraindications to endoscopy are a radiologic suspicion of perforation or supraglottic or epiglottic burns with edema, which may be a harbinger of airway obstruction, therefore indicating endotracheal intubation or tracheostomy. A third degree burn of the hypopharynx is a further contraindication for endoscopy[22].
Endoscopic classification[8] is important for prognosis and management (Table 3). Generally, grade 0 and 1 lesions do not develop delayed sequels, such as esophageal strictures or gastric outlet obstruction, whose incidence increases with the severity of the lesion. Additionally, the degree of esophageal injury at endoscopy is an accurate predictor of systemic complications and death, with each increased injury grade correlated with a 9-fold increase in morbidity and mortality[14]. Emergency surgery can be planned according to the endoscopic degree of burn, though an isolated black eschar does not always indicate full-thickness injury and the need for immediate surgical treatment: such patients may deserve further evaluation and careful observation. Recently, some concerns have been raised about the correlation between endoscopic findings and the extent of necrosis[39]: gastrectomy was considered unnecessary at laparotomy in 12% of patients with gastric injuries staged 3b at endoscopy, while the decision to perform esophagectomy based exclusively on endoscopic findings led to unnecessary esophagectomy in 15% of cases[52], suggesting the need for better criteria to improve patient selection for emergency surgery.
Table 3.
Endoscopic classification of caustic injuries
| Grade | Features |
| Grade 0 | Normal |
| Grade 1 | Superficial mucosal edema and erythema |
| Grade 2 | Mucosal and submucosal ulcerations |
| Grade 2A | Superficial ulcerations, erosions, exudates |
| Grade 2B | Deep discrete or circumferential ulcerations |
| Grade 3 | Transmural ulcerations with necrosis |
| Grade 3A | Focal necrosis |
| Grade 3B | Extensive necrosis |
| Grade 4 | Perforations |
Reproduced from Zargar et al[14].
MANAGEMENT
Acute management
Immediate treatment is usually conservative, as the definitive extent of the injury is determined within minutes after ingestion. Hemodynamic stabilization and adequacy of the patient’s airway are priorities. If the airway is unstable, fiberoptic laryngoscopy allows intubation under direct visualization, avoiding ‘‘blind’’ intubation with the risk of bleeding and additional injuries. In challenging patients, a surgical airway may be required. Gastric lavage and induced emesis are contraindicated for the risk of re-exposure to the corrosive agent and additional injury to the esophagus. The effectiveness of milk and water either as antidotes or to dilute the corrosive agents has never been proven. pH neutralization, with either a weak acid or base, is not recommended for fear of an exothermic reaction, which may increase the damage. Milk and activated charcoal are contraindicated because may obscure subsequent endoscopy. Nasogastric tubes may be applied to prevent vomiting and as stent in severe circumferential burns, but their validity has never be proven. In any case they should not be placed blindly because of the risk of esophageal perforation[53].
To date, the efficacy of proton-pump inhibitors and H2 blockers in minimizing esophageal injury by suppressing acid reflux has not been proven, though an impressive endoscopic healing after iv omeprazole infusion has been observed in a small prospective study[54].
The utility of corticosteroid is controversial. A meta-analysis of studies between 1991 and 2004, and an additional analysis of the literature over a longer period from 1956 to 2006 did not find any benefit of steroid administration in terms of stricture prevention. Steroids are usually reserved for patients with symptoms involving the airway[55,56].
The administration of broad-spectrum antibiotics is usually advised mainly if corticosteroids are initiated, as well as if lung involvement is identified[53,57].
Patients whose injuries are graded 1 and 2A are permitted oral intake and discharged within days with antacid therapy. In more severe cases (grade 2 or 3), observation in an intensive care unit and adequate nutritional support is required.
Early surgery
Patients with clinical or radiological evidence of perforation require immediate laparotomy, usually followed by esophagectomy, cervical esophagostomy, frequently concomitant gastrectomy and even more extensive resections, and jejunostomy feeding[58-60]. Some patients without features of perforation at admission may later develop necrosis, perforation and massive bleeding with disastrous results. Indications for emergency surgery rely more often on clinical grounds than on radiological findings; in the presence of doubtful clinical features a decision to perform laparotomy is likely more advantageous for patients than a conservative attitude especially in patients who ingested large amounts of corrosive substances[60].
Laboratory and endoscopic criteria for emergency surgery have been suggested, including disseminated intravascular coagulation, renal failure, acidosis and third degree esophageal burns[58,61]. Unfortunately, these are often late findings and surgery may improve mortality and morbidity in grade 3A injuries only[14].
Severe injuries of the stomach at endoscopy require careful monitoring with a low threshold for laparotomy. At surgery, a gastrotomy allows an accurate evaluation of the extent of damage, since mucosal (and transmural) necrosis may be more extensive than what is apparent from the serosal side. There is no role for procedures such as closure of a perforation. Conservative management of severe gastric injuries at laparotomy, with partial or total conservation of the stomach, has been recently advocated by some in the absence of clinical and biological signs of severity[62].
The need to perform surgery for caustic injuries has a persistent long-term negative impact both on survival and functional outcome. Moreover, esophageal resection per se, is an independent negative predictor of survival after emergency surgery[52].
Laparoscopy has been proposed when gastric perforation is highly suspected[63], but the mini-invasive approach has two caveats: unless in very expert hands, it is not a substitute for a comprehensive abdominal exploration, particularly in the posterior aspects of the stomach and duodenum, and it can extend the operative time excessively in a situation where time is a major determinant of outcome. However, it might be considered a useful tool when the stomach cannot be evaluated by endoscopy. Some authors have proposed routine laparoscopic examination in all injuries of second degree or greater[63,64] but the experience is still limited and laparoscopy may be neither feasible nor helpful in such dramatic circumstances.
All injured organs must be resected, if possible, during the first operation. Minimal resection followed by a planned second-look procedure is not recommended. However, secondary extension of caustic burns is unpredictable and re-exploration is indicated when in doubt. An extended resection to adjacent abdominal organs, even the pancreas, does not necessarily carry a prohibitive risk of death in referral centers[60], but an extensive colon resection may compromise future reconstruction and require vascular surgery for atypical transplants. A massive intestinal necrotic injury represents a reasonable limit for resection.
Emergency surgery may be required in the case of severe, uncontrolled late gastric bleeding, usually 1-2 wk after ingestion. Total gastrectomy may be necessary. In duodenal hemorrhages, under-running of the bleeding vessel through a duodenotomy is advised[29].
Acute surgery is quite exceptional in the pediatric population and most authors recommend exhausting all resources to try to preserve the child’s native esophagus[25].
Late sequelae
Following a grade 2B and a grade 3 esophageal burn, stricture incidence may be 71%[14] and 100%, respectively[45,53]. Strictures usually develop within 8 wk after the ingestion in 80% of patients, but it can happen as early as after 3 wk or as late as after 1 year. Obviously, ingestion of powerful caustic substances (e.g., sodium hydroxide) is followed by severe, long-standing strictures and dramatically altered esophageal motility[65].
Late sequelae of corrosive gastric injury include intractable pain, gastric outlet obstruction, late achlorhydria, protein-losing gastroenteropathy, mucosal metaplasia and development of carcinoma[66]. Gastric outlet obstruction has an incidence of 5%[67], mainly in the prepyloric area, where prolonged contact with the antral mucosa due to pyloric spasms and to resulting pooling of the caustic agent in this region[55] usually results in stricture in more than 60% of patients[68]. When the volume of the corrosive substance ingested is large, the entire stomach is scarred leading to a diffusely contracted stomach.
Stricture prevention
Steroids: Systemic administration of steroids seems ineffective in preventing strictures[55,56], especially in patients with 3rd degree esophageal burns. Intralesional triamcinolone injections have been proposed to prevent strictures[69], but optimal dose, frequency, and best application techniques are still to be defined[70].
Antibiotics: Though an old study reports a marked decrease in stricture formation with the use of antibiotics[71], no prospective trial evaluated their utility, and their value in the setting of caustic ingestion, in the absence of concomitant infection, is unknown[18]. There is a consensus that patients treated with steroids should also be treated with antibiotics, but prophylactic antibiotics to prevent strictures, in the absence of steroid therapy, has not been advocated[72].
Nasogastric tube: Though a nasogastric tube may be helpful to ensure patency of the esophageal lumen, the tube itself can contribute to the development of long strictures and routine use is not uniformly recommended[22]. Any esophageal catheterization may be a nidus for infection and nasogastric placement may worsen gastroesophageal reflux in this patient population, with a consequent delay in mucosal healing. However, enteral nutrition through a nasogastric tube has been demonstrated to be as effective as jejunostomy feeding in maintaining nutrition in such patients, with a similar rate of stricture development[73]. Moreover, positioning a nasogastric tube has the advantage of providing a lumen for dilatation should a tight stricture develops. Therefore, after caustic injuries the placement of a nasogastric tube may be considered, but the decision should be made with caution and done on a case-by-case basis.
Mitomycin C: Mitomycin C, a chemotherapeutic agent with DNA crosslinking activity, when injected or applied topically to the esophageal mucosa, may be valuable in preventing strictures, but this drug has deleterious adverse effects, especially if systemic absorption occurs across the intact mucosa[74]. A recent systematic review indicated encouraging results in the long term[75], but prospective studies are clearly mandatory to determine the most effective concentration, duration and frequency of application[76]. The theoretical risk of secondary long-term malignancy should also be taken into account[77].
Intraluminal stent: Specially designed silicone rubber[78] or, more recently, polyflex stents[79] have been found helpful in preventing stricture formation but the efficacy is less than 50%, with a high migration rate (25%). Patient selection remains a challenge and the development of hyperplastic tissue is a concern. Home-made polytetrafluoroethylene stents have shown promising results with a 72% efficacy[80] at 9-14 mo, similar to home-made silicone stents positioned by endoscopy[81] or through laparotomy[82] for 4-6 mo. Biodegradable stents (poly-L-lactide or polydioxanone) are under evaluation for benign strictures[83,84], with a 45% success rate at 53 mo in a patient population with only two caustic strictures, a migration rate of around 10%, and a significant hyperplastic tissue response. Experimentally, biodegradable stents were not able to prevent strictures in pigs after circumferential submucosal resection[85]. Moreover, cost and minimal experience in caustic strictures make the use of biodegradable devices questionable, especially in developing countries.
Other modalities for stricture prevention under evaluation: Intraperitoneal injection of 5-fluorouracil has been effective in preventing strictures experimentally[86]. Anti-oxidant treatment (vitamin E, H1 blocker, mast cell stabilizer, methylprednisolone) and phosphatidylcoline[87,88] inhibit collagen production and stricture formation by decreasing tissue hydroxyproline, the ultimate product of collagen degradation, but no human study is available. Octreotide and interferon-alfa-2b have been shown in animals to depress the fibrotic activity in the second phase of wound healing of the esophageal wall after a corrosive burn[89]. Cytokines have also been used experimentally with success to prevent stricture formation[90]. Until now, none of the above approaches, albeit appealing, has been tested in humans.
Stricture management
Endoscopic dilatation: Timely evaluation and dilatation of the stricture play a central role in achieving a good outcome[91]. Late management is usually associated with marked esophageal wall fibrosis and collagen deposition[5], which makes dilatation more complex. Maximal esophageal wall thickness, observed at CT scan, was associated with a higher number of sessions required for adequate dilatation[92], and recurrent strictures were significantly more frequent after delayed dilatation (Figure 4)[93-95]. Moreover, delayed presentation and treatment have been found to be strong predictors of future esophageal replacement[96]. This issue, which may entail different management strategies[3] for early or late patients, may be crucial in developing countries, where late presentations are more than 50%[2,97,98].
Figure 4.
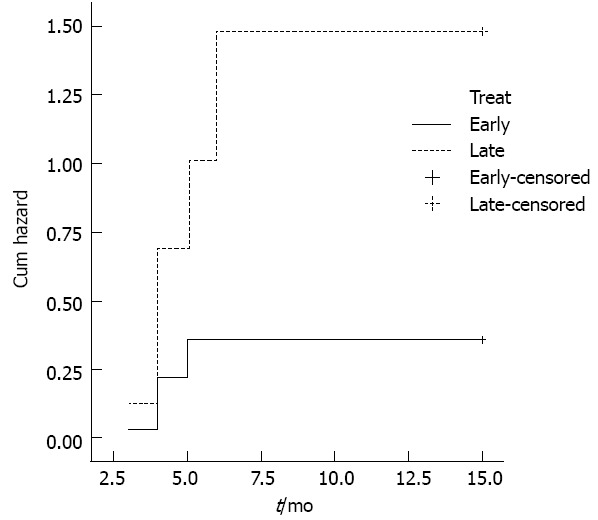
Significantly higher hazard of re-dilatation in patients submitted to late dilatation. P = 0.0008. Reproduced from Contini et al[97].
Dilatation can be carried out with balloon or bougies (usually Savary) without a clear advantage for each method[70]. However, the failure rate after pneumatic dilatation is higher in caustic ingestion-related strictures than in other benign strictures[99]; Savary bougies are considered more reliable than balloon dilators in consolidated and fibrotic strictures such as old caustic stenosis or in long, tortuous strictures[100,101], and may offer the operator the advantage of feeling the dilatation occurring under his hands[102]. Dilatation should be avoided from 7 to 21 d after ingestion for the risk of perforation, though early, prophylactic dilatation with bougienage has been reported to be safe and effective even in this period[43]. The perforation rate after dilatation of benign esophageal strictures varies between 0.1% and 0.4%[70], but for caustic strictures it fluctuates from 0.4% to 32.0%, dropping from 17.6% to 4.5% with increased experience[103]. The 5%-8% perforation rate after balloon dilatation[104] may be as high as 32% in caustic strictures[105]. Indeed, radiological intramural and well-contained transmural esophageal ruptures were observed in 30% of balloon dilatation procedures[106]. In addition, balloon inflation may cause either extrinsic mechanical compression of the trachea or obstruction at the endotracheal tube tip[107]. Therefore, the use of the balloon catheter in children entails careful intraoperative monitoring and likely requires greater endoscopic skill and experience than for Savary bougies. If these requirements are not met, as is often the case in developing countries, pneumatic dilatations will carry a considerable risk and then require extra caution, so that bougie dilatation is preferred.
The interval between dilatations varies from less than 1 to 2-3 wk and usually 3-4 sessions are considered sufficient for durable results, although the number of dilatations required may be unpredictable and quite high[103]. In challenging strictures, a nylon thread left between the nose and the gastrostomy maintains luminal access and facilitates further dilatations when an expert endoscopist is not available[108,109]. A cut-off value for unsuccessful dilatation treatment may be difficult to define, especially in developing countries, where alternative surgical options are not widely available.
A good nutritional state is crucial for a successful outcome, especially in children, and both an improvement in nutritional status and sustained esophageal patency should be considered reference points for a successful dilatation[3]. Changes in feeding practices may be required in order to maintain an adequate nutritional status[110]. In developing countries, delayed presentation and severity of strictures due to the more corrosive substances usually ingested, together with poor nursing and surgical care make this target quite challenging. In such a scenario, feeding by nasogastric tube for long periods may be tolerated with difficulty and a gastrostomy is more effective and often necessary to attain an acceptable nutritional state. Moreover, gastrostomy allows a retrograde approach for dilatation, which is usually easier and safer[111,112].
RISK OF CANCER
Esophageal neoplasms (both adenocarcinoma and squamous cell carcinoma) may develop as a late complication of caustic injury at a rate 1000-3000 times higher than expected in patients of a similar age[113] and have actually been reported only 1 year after ingestion[114]. The reported incidence ranges from 2% to 30%, with an interval from 1 to 3 decades after ingestion[53]. Cancer is most commonly observed at the areas of anatomic narrowing, and may be related to increased exposure to the caustic substance. Esophageal bypass surgery does not prevent the development of esophageal cancer following caustic ingestion[53]. The problem may be overestimated, in accordance with the low number of esophageal cancer reported in a large series with long-term follow-up[9,115,116], yet endoscopic screening is still recommended for patients following caustic ingestion. Moreover, the role of other confounding factors, such as alcohol abuse or smoking habit, should be considered[39].
DISMOTILITY
Orocecal transit time is prolonged mainly in patients with lower third esophageal involvement of the burn[65], probably related to autovagotomy due to vagal entrapment in the cicatrization process involving the lower third of the esophagus. Moreover, impaired vagal cholinergic transmission, possibly due to the same mechanism[117] can explain the increased fasting gallbladder volume and decreased gallbladder emptying found in patients after lower esophageal damage.
Gastric emptying time of liquids after caustic ingestion, was found to be significantly prolonged in patients with lower esophageal strictures, but not in upper-middle esophageal strictures, even in the absence of symptoms suggestive of gastric outlet obstruction or gastroparesis[118].
Late surgery
Surgery for non-responding esophageal strictures: When esophageal dilatation is not possible or fails to provide an adequate esophageal caliber in the long-term, esophageal replacement by retrosternal stomach or, preferably, right colonic interposition should be considered. Mortality and morbidity are low in expert hands[119,120]. The more demanding pharyngoesophageal strictures may be treated with acceptable results, provided considerable expertise is available[121]. The native esophagus can be left or removed. Though resection of the scarred esophagus may be performed without a substantial increase in morbidity and mortality compared to by-pass[120], a 13% incidence of esophageal cancer after by-pass[93], the risk of infected esophageal mucocele in 50% of the patients after 5 years[94], and the impossibility of endoscopic follow-up for cancer are all arguments favoring esophageal resection. Removal of the native esophagus seems advisable in children because of the risk of cancer in a long life period. Conversely, the doubled mortality rate (11.0% vs 5.9%) of resection vs by-pass[122], the possible damage to the trachea and laryngeal nerve, and the low reported incidence (3.2%) of esophageal malignancy, could support a conservative strategy. In children, reconstruction with gastroplasty seems easier, and more functional failures can be expected with coloplasty[123-125]. In developing countries, experienced pediatric surgical centers are not widely available and this should be considered before abandoning the conservative approach of dilatation.
Surgery for stomach injuries: The timing and type of elective surgery for gastric outlet obstruction is still controversial. Early surgery has been advised to decrease mortality and morbidity[67,126]. Conversely, elective surgery earlier than 3 mo has been considered risky because of poor nutritional state and the presence of adhesions and the edematous gastric wall[27]. Moreover, assessment of the limits of the gastric resection may be difficult, due to ongoing fibrosis. Endoscopic balloon dilatation and/or intralesional steroid injection have been proposed as alternatives[127,128]. However, endoscopic gastric dilatation should be considered a temporary substitute for surgical resection because gastric wall fibrosis usually diminishes the long-term functional result[129,130]. Moreover, although dilatation averts surgery in less than 50% of patients[127], perforation can occur in strictures longer than 15 mm[131]. Pyloroplasty has been recommended for moderate strictures[67], but progressive fibrosis causing recurrent stricture occurs frequently. Gastrojejunostomy is a safer alternative to gastric resection in the presence of extensive perigastric adhesion, an unhealthy duodenum, and poor general condition; marginal ulceration is rarely reported[27,132] possibly due to physiologic antrectomy resulting from mucosal damage[66]. Partial gastric resection is preferred by many[133,134] for the long-term risk of malignant transformation, though the need for gastric resection as prophylaxis against future malignancy has been overstated in the literature[29]. Previous reports of gastric carcinomas after acid ingestion are usually old and limited[135,136]. Regular follow-up and surveillance endoscopy is a more reliable approach.
Late reconstructive surgery after emergency esophagectomy: When the stomach has been removed or shows chronic injuries, the use of a gastric tube for esophageal reconstruction is obviously precluded. Reconstruction is probably advisable at the end of the evolving scarring process, usually after 6 mo, although the optimal timing of reconstruction has been reported from 2 mo to years[94,137,138]. The functional success rate after colon reconstruction at 5 years is 77% and the severity of the initial insult or a delay more than 6 mo, may strongly influence the outcome[119]. Coloplasty dysfunction is responsible for half of the failures, with an overall 70% success rate after revision surgery in expert hands. An emergency tracheostomy may have an adverse impact on the outcome of a colopharyngoplasty[139]. Secondary esophagocoloplasty should be considered with good results if intraoperative colon necrosis occurs at the time of primary reconstruction[140].
CONCLUSION
Ingestion of corrosive substances is increasingly reported in developing countries, due to lack of education and prevention. The relationship between symptoms and severity of injury may be vague, and patients should be carefully monitored, since esophageal or gastric perforations can occur at any time during the first 2 wk after ingestion. Endoscopy is considered a cornerstone in the diagnosis of corrosive ingestions, yet the indication for early endoscopy should likely be made on a case-by-case basis. Reported discrepancies between endoscopic findings and the extent of necrosis found at surgery suggest the need for better criteria to improve patient selection for emergency surgery. A CT scan may offer a promising role in assessing the evolution of the injury and impending perforations. In suicide attempts, mortality is still high and the need to perform emergency surgery for caustic injuries has a persistent long-term negative impact both on survival and functional outcome. However, timely and early surgery may be the only hope for patients with severe injuries, and a rather aggressive attitude should be considered in such patients.
Main late sequelae include esophageal strictures, often accompanied by undernourishment, especially in developing countries. The likelihood of a gastric outlet obstruction should always be kept in mind. The presence of severe GER and of esophageal dysmotility may worsen the prognosis. Stricture prevention by stents seems promising but the experience is still limited. Systemic corticosteroids offer no role. Endoscopic dilatation is usually successful in achieving a patent esophageal lumen, but in complex strictures several attempts must be carried out, and in such patients bougies may be preferred to balloon dilatation. A cut-off value for unsuccessful dilatation treatment may be difficult to define, especially in developing countries, where alternative surgical options are not widely available. Both an improvement in nutritional status and a sustained esophageal patency should be considered reference points for a successful dilatation. Gastrostomy may be lifesaving in this perspective. Mortality and morbidity of esophageal replacement in patients not responding to dilatation are low in expert hands. The preservation of the native esophagus is still debated. When late reconstructive surgery is carried out after early emergency surgical treatment, the outcome is strongly influenced by coloplasty dysfunction, responsible for half of the failures. Risk of esophageal cancer after caustic ingestion might be overestimated, yet endoscopic screening is still recommended.
Footnotes
P- Reviewer Teoh AYB S- Editor Zhai HH L- Editor Cant MR E- Editor Li JY
References
- 1.Ghelardini C, Malmberg-Aiello P, Giotti A, Malcangio M, Bartolini A. Investigation into atropine-induced antinociception. Br J Pharmacol. 1990;101:49–54. doi: 10.1179/2046905512Y.00000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekpe EE, Ette V. Morbidity and mortality of caustic ingestion in rural children: experience in a new cardiothoracic surgery unit in Nigeria. ISRN Pediatr. 2012;2012:210632. doi: 10.5402/2012/210632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Contini S, Swarray-Deen A, Scarpignato C. Oesophageal corrosive injuries in children: a forgotten social and health challenge in developing countries. Bull World Health Organ. 2009;87:950–954. doi: 10.2471/BLT.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarioglu-Buke A, Corduk N, Atesci F, Karabul M, Koltuksuz U. A different aspect of corrosive ingestion in children: socio-demographic characteristics and effect of family functioning. Int J Pediatr Otorhinolaryngol. 2006;70:1791–1798. doi: 10.1016/j.ijporl.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Gumaste VV, Dave PB. Ingestion of corrosive substances by adults. Am J Gastroenterol. 1992;87:1–5. [PubMed] [Google Scholar]
- 6.Watson WA, Litovitz TL, Rodgers GC, Klein-Schwartz W, Reid N, Youniss J, Flanagan A, Wruk KM. 2004 Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2005;23:589–666. doi: 10.1016/j.ajem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Zargar SA, Kochhar R, Nagi B, Mehta S, Mehta SK. Ingestion of corrosive acids. Spectrum of injury to upper gastrointestinal tract and natural history. Gastroenterology. 1989;97:702–707. [PubMed] [Google Scholar]
- 8.Havanond C. Is there a difference between the management of grade 2b and 3 corrosive gastric injuries? J Med Assoc Thai. 2002;85:340–344. [PubMed] [Google Scholar]
- 9.Mamede RC, de Mello Filho FV. Ingestion of caustic substances and its complications. Sao Paulo Med J. 2001;119:10–15. doi: 10.1590/S1516-31802001000100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osman M, Russell J, Shukla D, Moghadamfalahi M, Granger DN. Responses of the murine esophageal microcirculation to acute exposure to alkali, acid, or hypochlorite. J Pediatr Surg. 2008;43:1672–1678. doi: 10.1016/j.jpedsurg.2008.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Triadafilopoulos G. Caustic ingestion in adults. Available from: http: //www.uptodate.com.
- 12.Arévalo-Silva C, Eliashar R, Wohlgelernter J, Elidan J, Gross M. Ingestion of caustic substances: a 15-year experience. Laryngoscope. 2006;116:1422–1426. doi: 10.1097/01.mlg.0000225376.83670.4d. [DOI] [PubMed] [Google Scholar]
- 13.Poley JW, Steyerberg EW, Kuipers EJ, Dees J, Hartmans R, Tilanus HW, Siersema PD. Ingestion of acid and alkaline agents: outcome and prognostic value of early upper endoscopy. Gastrointest Endosc. 2004;60:372–377. doi: 10.1016/S0016-5107(04)01722-5. [DOI] [PubMed] [Google Scholar]
- 14.Zargar SA, Kochhar R, Mehta S, Mehta SK. The role of fiberoptic endoscopy in the management of corrosive ingestion and modified endoscopic classification of burns. Gastrointest Endosc. 1991;37:165–169. doi: 10.1016/S0016-5107(91)70678-0. [DOI] [PubMed] [Google Scholar]
- 15.Mutaf O, Genç A, Herek O, Demircan M, Ozcan C, Arikan A. Gastroesophageal reflux: a determinant in the outcome of caustic esophageal burns. J Pediatr Surg. 1996;31:1494–1495. doi: 10.1016/S0022-3468(96)90163-3. [DOI] [PubMed] [Google Scholar]
- 16.Bautista A, Varela R, Villanueva A, Estevez E, Tojo R, Cadranel S. Motor function of the esophagus after caustic burn. Eur J Pediatr Surg. 1996;6:204–207. doi: 10.1055/s-2008-1066508. [DOI] [PubMed] [Google Scholar]
- 17.Günel E, Cağlayan F, Cağlayan O, Akillioğlu I. Reactive oxygen radical levels in caustic esophageal burns. J Pediatr Surg. 1999;34:405–407. doi: 10.1016/S0022-3468(99)90486-4. [DOI] [PubMed] [Google Scholar]
- 18.Salzman M, O’Malley RN. Updates on the evaluation and management of caustic exposures. Emerg Med Clin North Am. 2007;25:459–476; abstract x. doi: 10.1016/j.emc.2007.02.00. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman RS, Howland MA, Kamerow HN, Goldfrank LR. Comparison of titratable acid/alkaline reserve and pH in potentially caustic household products. J Toxicol Clin Toxicol. 1989;27:241–246. doi: 10.3109/1556365890899442. [DOI] [PubMed] [Google Scholar]
- 20.Gorman RL, Khin-Maung-Gyi MT, Klein-Schwartz W, Oderda GM, Benson B, Litovitz T, McCormick M, McElwee N, Spiller H, Krenzelok E. Initial symptoms as predictors of esophageal injury in alkaline corrosive ingestions. Am J Emerg Med. 1992;10:189–194. doi: 10.1016/0735-6757(92)90206-D. [DOI] [PubMed] [Google Scholar]
- 21.Haller JA, Andrews HG, White JJ, Tamer MA, Cleveland WW. Pathophysiology and management of acute corrosive burns of the esophagus: results of treatment in 285 children. J Pediatr Surg. 1971;6:578–584. doi: 10.1016/0022-3468(71)90382-4. [DOI] [PubMed] [Google Scholar]
- 22.Ramasamy K, Gumaste VV. Corrosive ingestion in adults. J Clin Gastroenterol. 2003;37:119–124. doi: 10.1097/00004836-200308000-0000580. [DOI] [PubMed] [Google Scholar]
- 23.Havanond C, Havanond P. Initial signs and symptoms as prognostic indicators of severe gastrointestinal tract injury due to corrosive ingestion. J Emerg Med. 2007;33:349–353. doi: 10.1016/j.jemermed.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 24.Gupta SK, Croffie JM, Fitzgerald JF. Is esophagogastroduodenoscopy necessary in all caustic ingestions? J Pediatr Gastroenterol Nutr. 2001;32:50–53. doi: 10.1097/00005176-200101000-0001. [DOI] [PubMed] [Google Scholar]
- 25.Gaudreault P, Parent M, McGuigan MA, Chicoine L, Lovejoy FH. Predictability of esophageal injury from signs and symptoms: a study of caustic ingestion in 378 children. Pediatrics. 1983;71:767–770. [PubMed] [Google Scholar]
- 26.Zargar SA, Kochhar R, Nagi B, Mehta S, Mehta SK. Ingestion of strong corrosive alkalis: spectrum of injury to upper gastrointestinal tract and natural history. Am J Gastroenterol. 1992;87:337–341. [PubMed] [Google Scholar]
- 27.Chaudhary A, Puri AS, Dhar P, Reddy P, Sachdev A, Lahoti D, Kumar N, Broor SL. Elective surgery for corrosive-induced gastric injury. World J Surg. 1996;20:703–706; discussion 706. doi: 10.1007/s002689900107. [DOI] [PubMed] [Google Scholar]
- 28.Ceylan H, Ozokutan BH, Gündüz F, Gözen A. Gastric perforation after corrosive ingestion. Pediatr Surg Int. 2011;27:649–653. doi: 10.1007/s00383-010-2739-6. [DOI] [PubMed] [Google Scholar]
- 29.Ananthakrishnan N, Parthasarathy G, Kate V. Acute corrosive injuries of the stomach: a single unit experience of thirty years. ISRN Gastroenterol. 2011;2011:914013. doi: 10.5402/2011/914013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng YL, Wu MH, Lin MY, Lai WW. Massive upper gastrointestinal bleeding after acid-corrosive injury. World J Surg. 2004;28:50–54. doi: 10.1007/s00268-003-6831-0. [DOI] [PubMed] [Google Scholar]
- 31.Turner A, Robinson P. Respiratory and gastrointestinal complications of caustic ingestion in children. Emerg Med J. 2005;22:359–361. doi: 10.1136/emj.2004.015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rigo GP, Camellini L, Azzolini F, Guazzetti S, Bedogni G, Merighi A, Bellis L, Scarcelli A, Manenti F. What is the utility of selected clinical and endoscopic parameters in predicting the risk of death after caustic ingestion? Endoscopy. 2002;34:304–310. doi: 10.1055/s-2002-23633. [DOI] [PubMed] [Google Scholar]
- 33.Cheng YJ, Kao EL. Arterial blood gas analysis in acute caustic ingestion injuries. Surg Today. 2003;33:483–485. doi: 10.1007/s10595-002-2523-y. [DOI] [PubMed] [Google Scholar]
- 34.Katzka DA. Caustic Injury to the Esophagus. Curr Treat Options Gastroenterol. 2001;4:59–66. doi: 10.1007/s11938-001-0047-x. [DOI] [PubMed] [Google Scholar]
- 35.Skucas J. Contrast media. In: Gore R, Levine M, Laufer I, editors. Textbook of Gastrointestinal Radiology. Philadelphia: WB Saunders; 2000. pp. 2–14. [Google Scholar]
- 36.Chiu HM, Lin JT, Huang SP, Chen CH, Yang CS, Wang HP. Prediction of bleeding and stricture formation after corrosive ingestion by EUS concurrent with upper endoscopy. Gastrointest Endosc. 2004;60:827–833. doi: 10.1016/s0016-5107(04)02031-0. [DOI] [PubMed] [Google Scholar]
- 37.Kamijo Y, Kondo I, Kokuto M, Kataoka Y, Soma K. Miniprobe ultrasonography for determining prognosis in corrosive esophagitis. Am J Gastroenterol. 2004;99:851–854. doi: 10.1111/j.1572-0241.2004.30217.x. [DOI] [PubMed] [Google Scholar]
- 38.Rana SS, Bhasin DK, Nanda M, Siyad I, Gupta R, Kang M, Nagi B, Singh K. Endoscopic transpapillary drainage for external fistulas developing after surgical or radiological pancreatic interventions. J Gastroenterol Hepatol. 2010;25:1087–1092. doi: 10.1111/j.1440-1746.2010.06314.x. [DOI] [PubMed] [Google Scholar]
- 39.Keh SM, Onyekwelu N, McManus K, McGuigan J. Corrosive injury to upper gastrointestinal tract: Still a major surgical dilemma. World J Gastroenterol. 2006;12:5223–5228. doi: 10.3748/wjg.v12.i32.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryu HH, Jeung KW, Lee BK, Uhm JH, Park YH, Shin MH, Kim HL, Heo T, Min YI. Caustic injury: can CT grading system enable prediction of esophageal stricture? Clin Toxicol (Phila) 2010;48:137–142. doi: 10.3109/15563650903585929. [DOI] [PubMed] [Google Scholar]
- 41.Isbister GK, Page CB. Early endoscopy or CT in caustic injuries: a re-evaluation of clinical practice. Clin Toxicol (Phila) 2011;49:641–642. doi: 10.3109/15563650.2011.604035. [DOI] [PubMed] [Google Scholar]
- 42.Previtera C, Giusti F, Guglielmi M. Predictive value of visible lesions (cheeks, lips, oropharynx) in suspected caustic ingestion: may endoscopy reasonably be omitted in completely negative pediatric patients? Pediatr Emerg Care. 1990;6:176–178. doi: 10.1097/00006565-199009000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Tiryaki T, Livanelioğlu Z, Atayurt H. Early bougienage for relief of stricture formation following caustic esophageal burns. Pediatr Surg Int. 2005;21:78–80. doi: 10.1007/s00383-004-1331-3. [DOI] [PubMed] [Google Scholar]
- 44.Contini S, Tesfaye M, Picone P, Pacchione D, Kuppers B, Zambianchi C, Scarpignato C. Corrosive esophageal injuries in children. A shortlived experience in Sierra Leone. Int J Pediatr Otorhinolaryngol. 2007;71:1597–1604. doi: 10.1016/j.ijporl.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Baskin D, Urganci N, Abbasoğlu L, Alkim C, Yalçin M, Karadağ C, Sever N. A standardised protocol for the acute management of corrosive ingestion in children. Pediatr Surg Int. 2004;20:824–828. doi: 10.1007/s00383-004-1294-4. [DOI] [PubMed] [Google Scholar]
- 46.Aronow SP, Aronow HD, Blanchard T, Czinn S, Chelimsky G. Hair relaxers: a benign caustic ingestion? J Pediatr Gastroenterol Nutr. 2003;36:120–125. doi: 10.1097/00005176-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 47.Betalli P, Falchetti D, Giuliani S, Pane A, Dall’Oglio L, de Angelis GL, Caldore M, Romano C, Gamba P, Baldo V. Caustic ingestion in children: is endoscopy always indicated? The results of an Italian multicenter observational study. Gastrointest Endosc. 2008;68:434–439. doi: 10.1016/j.gie.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Temiz A, Oguzkurt P, Ezer SS, Ince E, Hicsonmez A. Predictability of outcome of caustic ingestion by esophagogastroduodenoscopy in children. World J Gastroenterol. 2012;18:1098–1103. doi: 10.3748/wjg.v18.i10.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christesen HB. Prediction of complications following unintentional caustic ingestion in children. Is endoscopy always necessary? Acta Paediatr. 1995;84:1177–1182. doi: 10.1111/j.1651-2227.1995.tb13520.x. [DOI] [PubMed] [Google Scholar]
- 50.Celik B, Nadir A, Sahin E, Kaptanoglu M. Is esophagoscopy necessary for corrosive ingestion in adults? Dis Esophagus. 2009;22:638–641. doi: 10.1111/j.1442-2050.2009.00987.x. [DOI] [PubMed] [Google Scholar]
- 51.Núñez O, González-Asanza C, de la Cruz G, Clemente G, Bañares R, Cos E, Menchén P. Study of predictive factors of severe digestive lesions due to caustics ingestion. Med Clin (Barc) 2004;123:611–614. doi: 10.1016/s0025-7753(04)74617-5. [DOI] [PubMed] [Google Scholar]
- 52.Chirica M, Resche-Rigon M, Bongrand NM, Zohar S, Halimi B, Gornet JM, Sarfati E, Cattan P. Surgery for caustic injuries of the upper gastrointestinal tract. Ann Surg. 2012;256:994–1001. doi: 10.1097/SLA.0b013e3182583fb2. [DOI] [PubMed] [Google Scholar]
- 53.Kay M, Wyllie R. Caustic ingestions in children. Curr Opin Pediatr. 2009;21:651–654. doi: 10.1097/MOP.0b013e32832e2764. [DOI] [PubMed] [Google Scholar]
- 54.Cakal B, Akbal E, Köklü S, Babalı A, Koçak E, Taş A. Acute therapy with intravenous omeprazole on caustic esophageal injury: a prospective case series. Dis Esophagus. 2013;26:22–26. doi: 10.1111/j.1442-2050.2011.01319.x. [DOI] [PubMed] [Google Scholar]
- 55.Pelclová D, Navrátil T. Do corticosteroids prevent oesophageal stricture after corrosive ingestion? Toxicol Rev. 2005;24:125–129. doi: 10.2165/00139709-200524020-00006. [DOI] [PubMed] [Google Scholar]
- 56.Fulton JA, Hoffman RS. Steroids in second degree caustic burns of the esophagus: a systematic pooled analysis of fifty years of human data: 1956-2006. Clin Toxicol (Phila) 2007;45:402–408. doi: 10.1080/15563650701285420. [DOI] [PubMed] [Google Scholar]
- 57.Cheng HT, Cheng CL, Lin CH, Tang JH, Chu YY, Liu NJ, Chen PC. Caustic ingestion in adults: the role of endoscopic classification in predicting outcome. BMC Gastroenterol. 2008;8:31. doi: 10.1186/1471-230X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu MH, Lai WW. Surgical management of extensive corrosive injuries of the alimentary tract. Surg Gynecol Obstet. 1993;177:12–16. [PubMed] [Google Scholar]
- 59.Andreoni B, Farina ML, Biffi R, Crosta C. Esophageal perforation and caustic injury: emergency management of caustic ingestion. Dis Esophagus. 1997;10:95–100. doi: 10.1093/dote/10.2.95. [DOI] [PubMed] [Google Scholar]
- 60.Cattan P, Munoz-Bongrand N, Berney T, Halimi B, Sarfati E, Celerier M. Extensive abdominal surgery after caustic ingestion. Ann Surg. 2000;231:519–523. doi: 10.1097/00000658-200004000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brun JG, Celerier M, Koskas F, Dubost C. Blunt thorax oesophageal stripping: an emergency procedure for caustic ingestion. Br J Surg. 1984;71:698–700. doi: 10.1002/bjs.1800710918. [DOI] [PubMed] [Google Scholar]
- 62.Zerbib P, Voisin B, Truant S, Saulnier F, Vinet A, Chambon JP, Onimus T, Pruvot FR. The conservative management of severe caustic gastric injuries. Ann Surg. 2011;253:684–688. doi: 10.1097/SLA.0b013e31821110e8. [DOI] [PubMed] [Google Scholar]
- 63.Huscher CG, Mingoli A, Mereu A, Sgarzini G. Laparoscopy can be very effective in reducing mortality rate for caustic ingestion in suicide attempt. World J Surg. 2011;35:2363–2364; author reply 2365. doi: 10.1007/s00268-011-1120-9. [DOI] [PubMed] [Google Scholar]
- 64.Hugh TB, Kelly MD. Corrosive ingestion and the surgeon. J Am Coll Surg. 1999;189:508–522. doi: 10.1016/s1072-7515(99)00160-x. [DOI] [PubMed] [Google Scholar]
- 65.Genç A, Mutaf O. Esophageal motility changes in acute and late periods of caustic esophageal burns and their relation to prognosis in children. J Pediatr Surg. 2002;37:1526–1528. doi: 10.1053/jpsu.2002.36177. [DOI] [PubMed] [Google Scholar]
- 66.McAuley CE, Steed DL, Webster MW. Late sequelae of gastric acid injury. Am J Surg. 1985;149:412–415. doi: 10.1016/s0002-9610(85)80121-5. [DOI] [PubMed] [Google Scholar]
- 67.Ciftci AO, Senocak ME, Büyükpamukçu N, Hiçsönmez A. Gastric outlet obstruction due to corrosive ingestion: incidence and outcome. Pediatr Surg Int. 1999;15:88–91. doi: 10.1007/s003830050523. [DOI] [PubMed] [Google Scholar]
- 68.Gupta V, Wig JD, Kochhar R, Sinha SK, Nagi B, Doley RP, Gupta R, Yadav TD. Surgical management of gastric cicatrisation resulting from corrosive ingestion. Int J Surg. 2009;7:257–261. doi: 10.1016/j.ijsu.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 69.Kochhar R, Ray JD, Sriram PV, Kumar S, Singh K. Intralesional steroids augment the effects of endoscopic dilation in corrosive esophageal strictures. Gastrointest Endosc. 1999;49:509–513. doi: 10.1016/s0016-5107(99)70052-0. [DOI] [PubMed] [Google Scholar]
- 70.Siersema PD, de Wijkerslooth LR. Dilation of refractory benign esophageal strictures. Gastrointest Endosc. 2009;70:1000–1012. doi: 10.1016/j.gie.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Krey H. On the treatment of corrosive lesions in the oesophagus; an experimental study. Acta Otolaryngol Suppl. 1952;102:1–49. [PubMed] [Google Scholar]
- 72.Rao RB, Hoffman RS. Caustics and Batteries. In: Goldfrank LR, Norwalk CT, editors. Goldfrank’s Toxicologic Emergencies. Norwalk: Appleton and Lange; 1998. pp. 1399–1428. [Google Scholar]
- 73.Kochhar R, Poornachandra KS, Puri P, Dutta U, Sinha SK, Sethy PK, Wig JD, Nagi B, Singh K. Comparative evaluation of nasoenteral feeding and jejunostomy feeding in acute corrosive injury: a retrospective analysis. Gastrointest Endosc. 2009;70:874–880. doi: 10.1016/j.gie.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 74.Uhlen S, Fayoux P, Vachin F, Guimber D, Gottrand F, Turck D, Michaud L. Mitomycin C: an alternative conservative treatment for refractory esophageal stricture in children? Endoscopy. 2006;38:404–407. doi: 10.1055/s-2006-925054. [DOI] [PubMed] [Google Scholar]
- 75.Berger M, Ure B, Lacher M. Mitomycin C in the therapy of recurrent esophageal strictures: hype or hope? Eur J Pediatr Surg. 2012;22:109–116. doi: 10.1055/s-0032-1311695. [DOI] [PubMed] [Google Scholar]
- 76.Ortolan EP, Bustamante TF, Higa KL, Da Silva AP, Takegawa BK. The Best Moment to Use Mitomycin C in Caustic Esophagitis. Experimental Study Gastroint Endosc. 2011;73(Suppl 4):AB199–AB200. doi: 10.1016/j.gie.2011.03.268. [DOI] [Google Scholar]
- 77.Berkovits RN, Bos CE, Wijburg FA, Holzki J. Caustic injury of the oesophagus. Sixteen years experience, and introduction of a new model oesophageal stent. J Laryngol Otol. 1996;110:1041–1045. doi: 10.1017/s0022215100135716. [DOI] [PubMed] [Google Scholar]
- 78.De Peppo F, Zaccara A, Dall’Oglio L, Federici di Abriola G, Ponticelli A, Marchetti P, Lucchetti MC, Rivosecchi M. Stenting for caustic strictures: esophageal replacement replaced. J Pediatr Surg. 1998;33:54–57. doi: 10.1016/s0022-3468(98)90361-x. [DOI] [PubMed] [Google Scholar]
- 79.Broto J, Asensio M, Vernet JM. Results of a new technique in the treatment of severe esophageal stenosis in children: poliflex stents. J Pediatr Gastroenterol Nutr. 2003;37:203–206. doi: 10.1097/00005176-200308000-00024. [DOI] [PubMed] [Google Scholar]
- 80.Atabek C, Surer I, Demirbag S, Caliskan B, Ozturk H, Cetinkursun S. Increasing tendency in caustic esophageal burns and long-term polytetrafluorethylene stenting in severe cases: 10 years experience. J Pediatr Surg. 2007;42:636–640. doi: 10.1016/j.jpedsurg.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 81.Foschia F, De Angelis P, Torroni F, Romeo E, Caldaro T, di Abriola GF, Pane A, Fiorenza MS, De Peppo F, Dall’Oglio L. Custom dynamic stent for esophageal strictures in children. J Pediatr Surg. 2011;46:848–853. doi: 10.1016/j.jpedsurg.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 82.Wang RW, Zhou JH, Jiang YG, Fan SZ, Gong TQ, Zhao YP, Tan QY, Lin YD. Prevention of stricture with intraluminal stenting through laparotomy after corrosive esophageal burns. Eur J Cardiothorac Surg. 2006;30:207–211. doi: 10.1016/j.ejcts.2006.03.068. [DOI] [PubMed] [Google Scholar]
- 83.Tokar JL, Banerjee S, Barth BA, Desilets DJ, Kaul V, Kethi SR, Pedrosa MC, Pfau PR, Pleskow DK, Varadarajulu S, et al. Drug-eluting/biodegradable stents. Gastrointest Endosc. 2011;74:954–958. doi: 10.1016/j.gie.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 84.Repici A, Vleggaar FP, Hassan C, van Boeckel PG, Romeo F, Pagano N, Malesci A, Siersema PD. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: the BEST (Biodegradable Esophageal Stent) study. Gastrointest Endosc. 2010;72:927–934. doi: 10.1016/j.gie.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 85.Pauli EM, Schomisch SJ, Furlan JP, Marks AS, Chak A, Lash RH, Ponsky JL, Marks JM. Biodegradable esophageal stent placement does not prevent high-grade stricture formation after circumferential mucosal resection in a porcine model. Surg Endosc. 2012;26:3500–3508. doi: 10.1007/s00464-012-2373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duman L, Büyükyavuz BI, Altuntas I, Gökcimen A, Ceyhan L, Darici H, Aylak F, Tomruk O. The efficacy of single-dose 5-fluorouracil therapy in experimental caustic esophageal burn. J Pediatr Surg. 2011;46:1893–1897. doi: 10.1016/j.jpedsurg.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 87.Demirbilek S, Aydin G, Yücesan S, Vural H, Bitiren M. Polyunsaturated phosphatidylcholine lowers collagen deposition in a rat model of corrosive esophageal burn. Eur J Pediatr Surg. 2002;12:8–12. doi: 10.1055/s-2002-25082. [DOI] [PubMed] [Google Scholar]
- 88.Günel E, Cağlayan F, Cağlayan O, Canbilen A, Tosun M. Effect of antioxidant therapy on collagen synthesis in corrosive esophageal burns. Pediatr Surg Int. 2002;18:24–27. doi: 10.1007/s003830200005. [DOI] [PubMed] [Google Scholar]
- 89.Kaygusuz I, Celik O, Ozkaya O O, Yalçin S, Keleş E, Cetinkaya T. Effects of interferon-alpha-2b and octreotide on healing of esophageal corrosive burns. Laryngoscope. 2001;111:1999–2004. doi: 10.1097/00005537-200111000-00025. [DOI] [PubMed] [Google Scholar]
- 90.Berthet B, di Costanzo J, Arnaud C, Choux R, Assadourian R. Influence of epidermal growth factor and interferon gamma on healing of oesophageal corrosive burns in the rat. Br J Surg. 1994;81:395–398. doi: 10.1002/bjs.1800810325. [DOI] [PubMed] [Google Scholar]
- 91.Doğan Y, Erkan T, Cokuğraş FC, Kutlu T. Caustic gastroesophageal lesions in childhood: an analysis of 473 cases. Clin Pediatr (Phila) 2006;45:435–438. doi: 10.1177/0009922806289618. [DOI] [PubMed] [Google Scholar]
- 92.Lahoti D, Broor SL, Basu PP, Gupta A, Sharma R, Pant CS. Corrosive esophageal strictures: predictors of response to endoscopic dilation. Gastrointest Endosc. 1995;41:196–200. doi: 10.1016/S0016-5107(95)70337-3. [DOI] [PubMed] [Google Scholar]
- 93.Kim YT, Sung SW, Kim JH. Is it necessary to resect the diseased esophagus in performing reconstruction for corrosive esophageal stricture? Eur J Cardiothorac Surg. 2001;20:1–6. doi: 10.1016/S1010-7940(01)00747-3. [DOI] [PubMed] [Google Scholar]
- 94.Gerzic ZB, Knezevic JB, Milicevic MN, Jovanovic BK. Esophagocoloplasty in the management of postcorrosive strictures of the esophagus. Ann Surg. 1990;211:329–336. doi: 10.1097/00000658-199003000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pace F, Antinori S, Repici A. What is new in esophageal injury (infection, drug-induced, caustic, stricture, perforation)? Curr Opin Gastroenterol. 2009;25:372–379. doi: 10.1097/MOG.0b013e32832ad2e4. [DOI] [PubMed] [Google Scholar]
- 96.Panieri E, Rode H, Millar AJ, Cywes S. Oesophageal replacement in the management of corrosive strictures: when is surgery indicated? Pediatr Surg Int. 1998;13:336–340. doi: 10.1007/s003830050333. [DOI] [PubMed] [Google Scholar]
- 97.Contini S, Garatti M, Swarray-Deen A, Depetris N, Cecchini S, Scarpignato C. Corrosive oesophageal strictures in children: outcomes after timely or delayed dilatation. Dig Liver Dis. 2009;41:263–268. doi: 10.1016/j.dld.2008.07.319. [DOI] [PubMed] [Google Scholar]
- 98.Gün F, Abbasoğlu L, Celik A, Salman ET. Early and late term management in caustic ingestion in children: a 16-year experience. Acta Chir Belg. 2007;107:49–52. doi: 10.1080/00015458.2007.11680010. [DOI] [PubMed] [Google Scholar]
- 99.Sandgren K, Malmfors G. Balloon dilatation of oesophageal strictures in children. Eur J Pediatr Surg. 1998;8:9–11. doi: 10.1055/s-2008-1071110. [DOI] [PubMed] [Google Scholar]
- 100.Dall’Oglio L, De Angelis P. Commentary on “Esophageal endoscopic dilations”. J Pediatr Gastroenterol Nutr. 2012;54:716–717. doi: 10.1097/MPG.0b013e31824b174e. [DOI] [PubMed] [Google Scholar]
- 101.Lakhdar-Idrissi M, Khabbache K, Hida M. Esophageal endoscopic dilations. J Pediatr Gastroenterol Nutr. 2012;54:744–747. doi: 10.1097/MPG.0b013e31824b16b2. [DOI] [PubMed] [Google Scholar]
- 102.Shehata SM, Enaba ME. Endoscopic dilatation for benign oesophageal strictures in infants and toddlers: experience of an expectant protocol from North African tertiary centre. Afr J Paediatr Surg. 2012;9:187–192. doi: 10.4103/0189-6725.104717. [DOI] [PubMed] [Google Scholar]
- 103.Contini S, Scarpignato C, Rossi A, Strada G. Features and management of esophageal corrosive lesions in children in Sierra Leone: lessons learned from 175 consecutive patients. J Pediatr Surg. 2011;46:1739–1745. doi: 10.1016/j.jpedsurg.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 104.Lan LC, Wong KK, Lin SC, Sprigg A, Clarke S, Johnson PR, Tam PK. Endoscopic balloon dilatation of esophageal strictures in infants and children: 17 years’ experience and a literature review. J Pediatr Surg. 2003;38:1712–1715. doi: 10.1016/j.jpedsurg.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 105.Song HY, Han YM, Kim HN, Kim CS, Choi KC. Corrosive esophageal stricture: safety and effectiveness of balloon dilation. Radiology. 1992;184:373–378. doi: 10.1148/radiology.184.2.1620830. [DOI] [PubMed] [Google Scholar]
- 106.Doo EY, Shin JH, Kim JH, Song HY. Oesophageal strictures caused by the ingestion of corrosive agents: effectiveness of balloon dilatation in children. Clin Radiol. 2009;64:265–271. doi: 10.1016/j.crad.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 107.Gerçek A, Ay B, Dogan V, Kiyan G, Dagli T, Gogus Y. Esophageal balloon dilation in children: prospective analysis of hemodynamic changes and complications during general anesthesia. J Clin Anesth. 2007;19:286–289. doi: 10.1016/j.jclinane.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 108.Hawkins DB. Dilation of esophageal strictures: comparative morbidity of antegrade and retrograde methods. Ann Otol Rhinol Laryngol. 1988;97:460–465. doi: 10.1177/000348948809700505. [DOI] [PubMed] [Google Scholar]
- 109.Saleem MM. Acquired oesophageal strictures in children: emphasis on the use of string-guided dilatations. Singapore Med J. 2009;50:82–86. [PubMed] [Google Scholar]
- 110.Sánchez-Ramírez CA, Larrosa-Haro A, Vásquez Garibay EM, Larios-Arceo F. Caustic ingestion and oesophageal damage in children: Clinical spectrum and feeding practices. J Paediatr Child Health. 2011;47:378–380. doi: 10.1111/j.1440-1754.2010.01984.x. [DOI] [PubMed] [Google Scholar]
- 111.Bueno R, Swanson SJ, Jaklitsch MT, Lukanich JM, Mentzer SJ, Sugarbaker DJ. Combined antegrade and retrograde dilation: a new endoscopic technique in the management of complex esophageal obstruction. Gastrointest Endosc. 2001;54:368–372. doi: 10.1067/mge.2001.117517. [DOI] [PubMed] [Google Scholar]
- 112.Mukherjee K, Cash MP, Burkey BB, Yarbrough WG, Netterville JL, Melvin WV. Antegrade and retrograde endoscopy for treatment of esophageal stricture. Am Surg. 2008;74:686–687; discussion 688. [PubMed] [Google Scholar]
- 113.Kiviranta NK. Corrosive carcinoma of the esophagus. Acta Otolaryngol. 1952;102:1–9. [Google Scholar]
- 114.Jain R, Gupta S, Pasricha N, Faujdar M, Sharma M, Mishra P. ESCC with metastasis in the young age of caustic ingestion of shortest duration. J Gastrointest Cancer. 2010;41:93–95. doi: 10.1007/s12029-009-9121-8. [DOI] [PubMed] [Google Scholar]
- 115.Marchand P. Caustic strictures of the oesophagus. Thorax. 1955;10:171–181. doi: 10.1136/thx.10.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carver GM, Sealy WC, Dillon ML. Management of alkali burns of the esophagus. J Am Med Assoc. 1956;160:1447–1450. doi: 10.1001/jama.1956.02960520009003. [DOI] [PubMed] [Google Scholar]
- 117.Khan BA, Kochhar R, Nagi B, Raja K, Singh K. Gall bladder emptying in patients with corrosive-induced esophageal strictures. Dig Dis Sci. 2005;50:111–115. doi: 10.1007/s10620-005-1287-8. [DOI] [PubMed] [Google Scholar]
- 118.Mittal BR, Kochhar R, Shankar R, Bhattacharya A, Solanki K, Nagi B. Delayed gastric emptying in patients with caustic ingestion. Nucl Med Commun. 2008;29:782–785. doi: 10.1097/MNM.0b013e328302f4b9. [DOI] [PubMed] [Google Scholar]
- 119.Chirica M, Veyrie N, Munoz-Bongrand N, Zohar S, Halimi B, Celerier M, Cattan P, Sarfati E. Late morbidity after colon interposition for corrosive esophageal injury: risk factors, management, and outcome. A 20-years experience. Ann Surg. 2010;252:271–280. doi: 10.1097/SLA.0b013e3181e8fd40. [DOI] [PubMed] [Google Scholar]
- 120.Javed A, Pal S, Dash NR, Sahni P, Chattopadhyay TK. Outcome following surgical management of corrosive strictures of the esophagus. Ann Surg. 2011;254:62–66. doi: 10.1097/SLA.0b013e3182125ce7. [DOI] [PubMed] [Google Scholar]
- 121.Ananthakrishnan N, Kate V, Parthasarathy G. Therapeutic options for management of pharyngoesophageal corrosive strictures. J Gastrointest Surg. 2011;15:566–575. doi: 10.1007/s11605-011-1454-5. [DOI] [PubMed] [Google Scholar]
- 122.Gupta NM, Gupta R. Transhiatal esophageal resection for corrosive injury. Ann Surg. 2004;239:359–363. doi: 10.1097/01.sla.0000114218.48318.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arul GS, Parikh D. Oesophageal replacement in children. Ann R Coll Surg Engl. 2008;90:7–12. doi: 10.1308/003588408X242222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cowles RA, Coran AG. Gastric transposition in infants and children. Pediatr Surg Int. 2010;26:1129–1134. doi: 10.1007/s00383-010-2736-9. [DOI] [PubMed] [Google Scholar]
- 125.Erdoğan E, Eroğlu E, Tekant G, Yeker Y, Emir H, Sarimurat N, Yeker D. Management of esophagogastric corrosive injuries in children. Eur J Pediatr Surg. 2003;13:289–293. doi: 10.1055/s-2003-43581. [DOI] [PubMed] [Google Scholar]
- 126.Tseng YL, Wu MH, Lin MY, Lai WW. Early surgical correction for isolated gastric stricture following acid corrosion injury. Dig Surg. 2002;19:276–280. doi: 10.1159/000064582. [DOI] [PubMed] [Google Scholar]
- 127.Temiz A, Oguzkurt P, Ezer SS, Ince E, Gezer HO, Hicsonmez A. Management of pyloric stricture in children: endoscopic balloon dilatation and surgery. Surg Endosc. 2012;26:1903–1908. doi: 10.1007/s00464-011-2124-0. [DOI] [PubMed] [Google Scholar]
- 128.Kochhar R, Sriram PV, Ray JD, Kumar S, Nagi B, Singh K. Intralesional steroid injections for corrosive induced pyloric stenosis. Endoscopy. 1998;30:734–736. doi: 10.1055/s-2007-1001400. [DOI] [PubMed] [Google Scholar]
- 129.Dumont O, Queneau PE, Bernard G, Berger F, Paliard P. Mid-term failure of balloon dilatation treatment of antral stenosis induced by caustics. Gastroenterol Clin Biol. 1995;19:302–304. [PubMed] [Google Scholar]
- 130.Tekant G, Eroğlu E, Erdoğan E, Yeşildağ E, Emir H, Büyükünal C, Yeker D. Corrosive injury-induced gastric outlet obstruction: a changing spectrum of agents and treatment. J Pediatr Surg. 2001;36:1004–1007. doi: 10.1053/jpsu.2001.24725. [DOI] [PubMed] [Google Scholar]
- 131.Kochhar R, Dutta U, Sethy PK, Singh G, Sinha SK, Nagi B, Wig JD, Singh K. Endoscopic balloon dilation in caustic-induced chronic gastric outlet obstruction. Gastrointest Endosc. 2009;69:800–805. doi: 10.1016/j.gie.2008.05.056. [DOI] [PubMed] [Google Scholar]
- 132.Ozcan C, Ergün O, Sen T, Mutaf O. Gastric outlet obstruction secondary to acid ingestion in children. J Pediatr Surg. 2004;39:1651–1653. doi: 10.1016/j.jpedsurg.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 133.Sarfati E, Gossot D, Assens P, Celerier M. Management of caustic ingestion in adults. Br J Surg. 1987;74:146–148. doi: 10.1002/bjs.1800740225. [DOI] [PubMed] [Google Scholar]
- 134.Agarwal S, Sikora SS, Kumar A, Saxena R, Kapoor VK. Surgical management of corrosive strictures of stomach. Indian J Gastroenterol. 2004;23:178–180. [PubMed] [Google Scholar]
- 135.O’donnell CH, Abbott WE, Hirshfeld JW. Surgical treatment of corrosive gastritis. Am J Surg. 1949;78:251–255. doi: 10.1016/0002-9610(49)90339-6. [DOI] [PubMed] [Google Scholar]
- 136.Eaton H, Tennekoon GE. Squamous carcinoma of the stomach following corrosive acid burns. Br J Surg. 1972;59:382–387. doi: 10.1002/bjs.1800590514. [DOI] [PubMed] [Google Scholar]
- 137.Bothereau H, Munoz-Bongrand N, Lambert B, Montemagno S, Cattan P, Sarfati E. Esophageal reconstruction after caustic injury: is there still a place for right coloplasty? Am J Surg. 2007;193:660–664. doi: 10.1016/j.amjsurg.2006.08.074. [DOI] [PubMed] [Google Scholar]
- 138.Knezević JD, Radovanović NS, Simić AP, Kotarac MM, Skrobić OM, Konstantinović VD, Pesko PM. Colon interposition in the treatment of esophageal caustic strictures: 40 years of experience. Dis Esophagus. 2007;20:530–534. doi: 10.1111/j.1442-2050.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- 139.Tettey M, Edwin F, Aniteye E, Tamatey M, Entsua-Mensah K, Ofosu-Appiah E, Frimpong-Boateng K. Colopharyngoplasty for intractable caustic pharyngoesophageal strictures in an indigenous African community--adverse impact of concomitant tracheostomy on outcome. Interact Cardiovasc Thorac Surg. 2011;12:213–217. doi: 10.1510/icvts.2010.241836. [DOI] [PubMed] [Google Scholar]
- 140.Chirica M, Vuarnesson H, Zohar S, Faron M, Halimi B, Munoz Bongrand N, Cattan P, Sarfati E. Similar outcomes after primary and secondary esophagocoloplasty for caustic injuries. Ann Thorac Surg. 2012;93:905–912. doi: 10.1016/j.athoracsur.2011.12.054. [DOI] [PubMed] [Google Scholar]


