Abstract
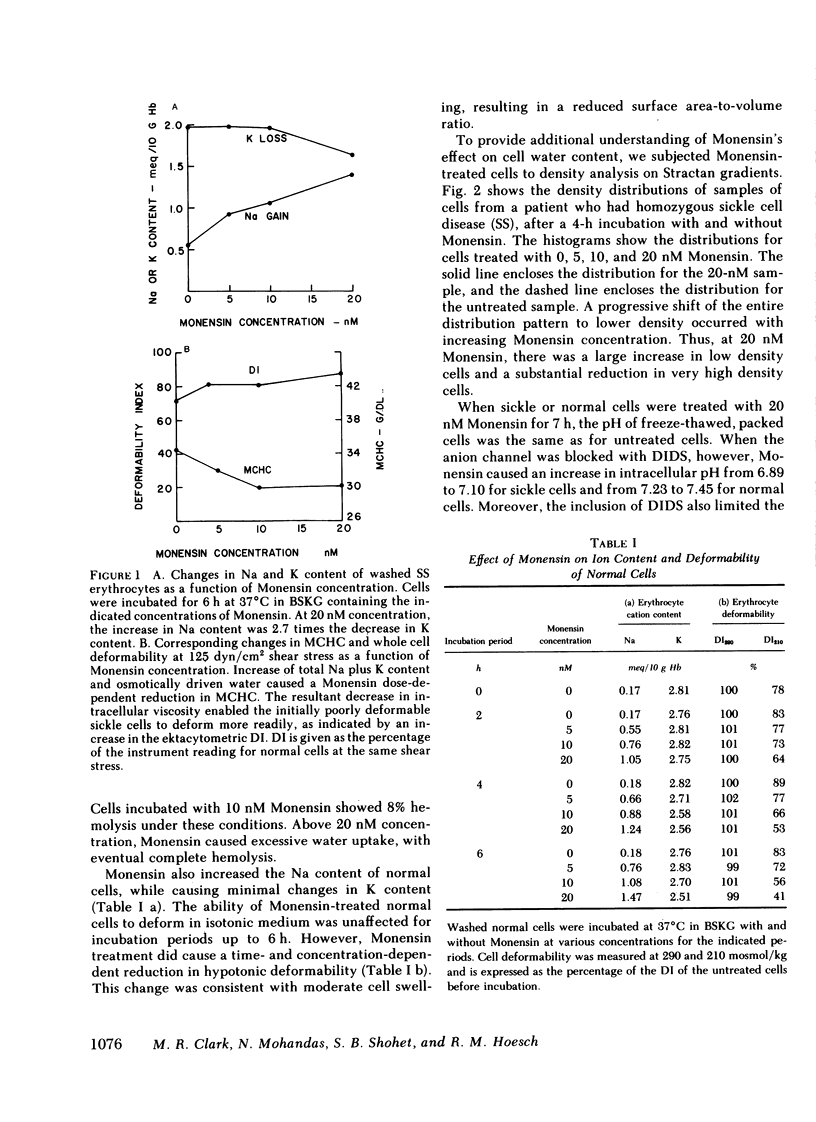
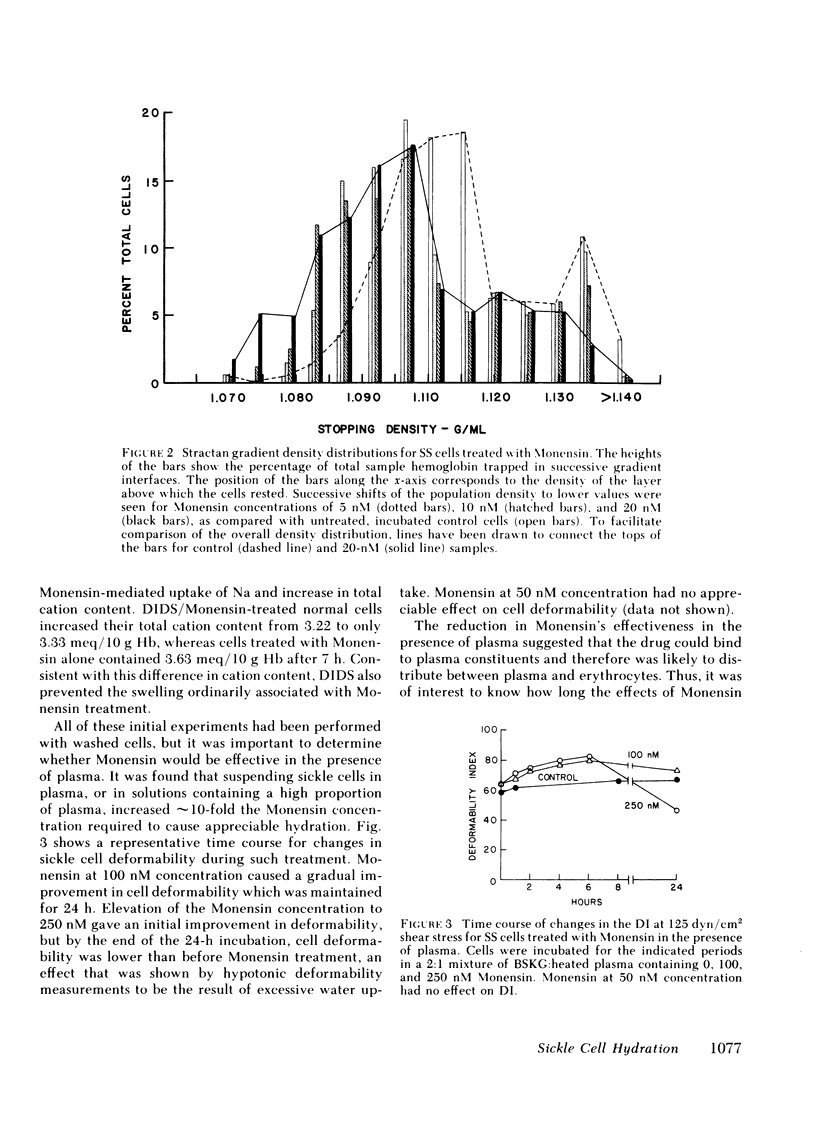
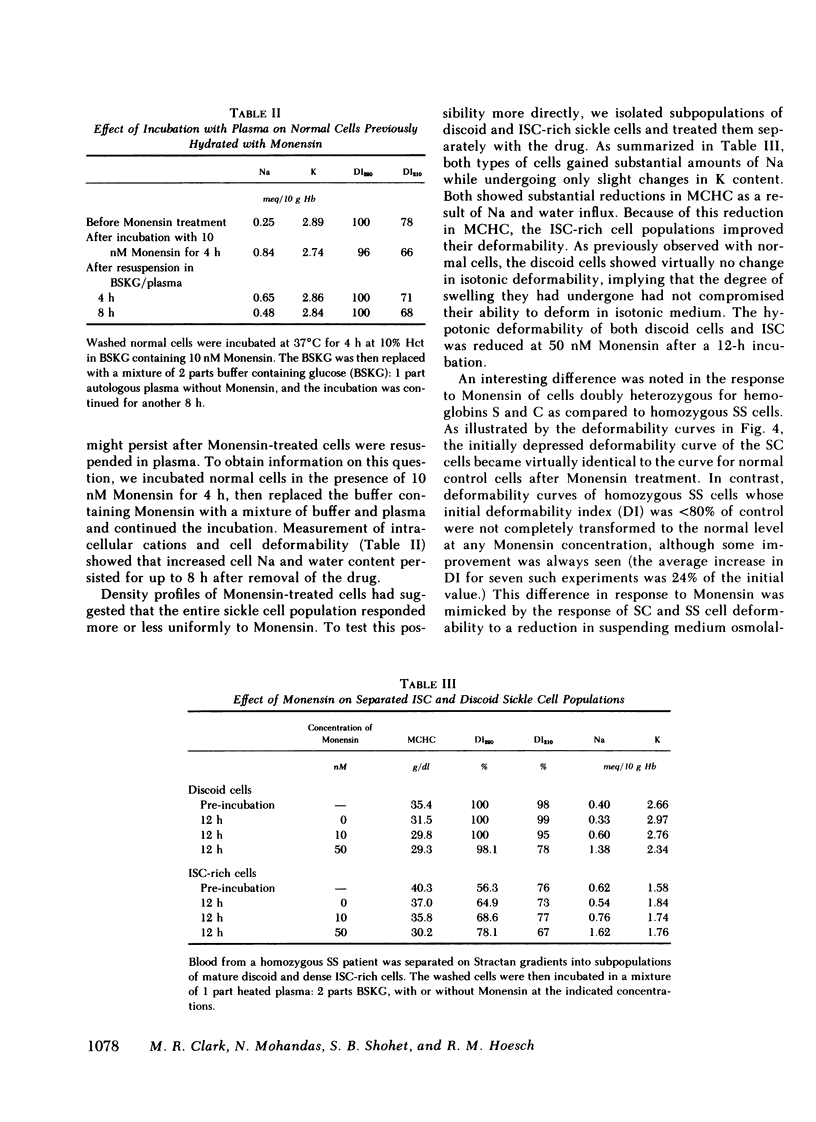
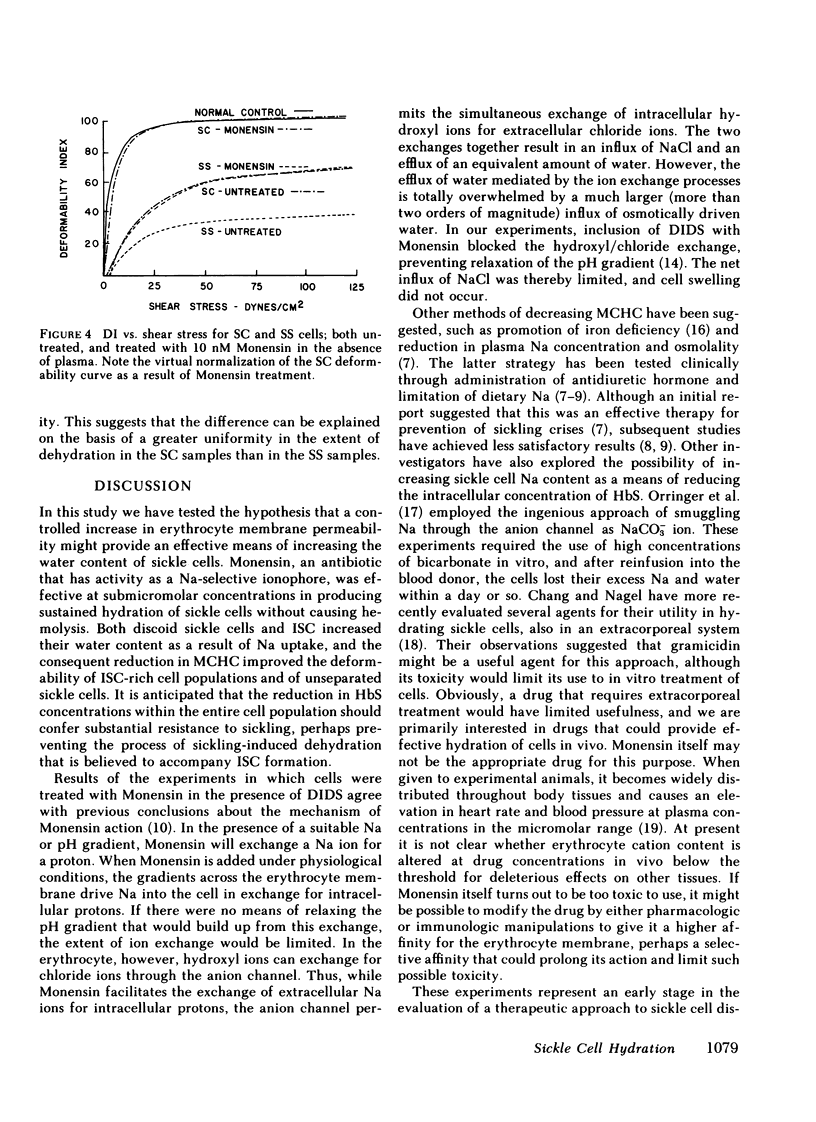
Mean cell hemoglobin concentration (MCHC) is thought to have an important influence in sickle cell disease, both through the strong dependence of sickling rates on hemoglobin S concentration, and through the profoundly limiting effect of high MCHC on the rheologic competence of oxygenated, irreversibly sickled cells (ISC). Recent studies have tested the ability of antidiuretic hormone to reduce sickle cell MCHC by reducing plasma sodium (Na) and osmolality. An alternative means of reducing MCHC is to elevate intracellular cation content, rather than to depress extracellular cation concentration. In an effort to do this, we have treated sickle cells with Monensin, an antibiotic that selectively enhances membrane Na permeability. At submicromolar concentrations, Monensin substantially reduced the MCHC of whole sickle blood and isolated ISC, causing an improvement in cell deformability. Monensin's effectiveness in producing a controlled increase in erythrocyte water content suggests that agents that selectively increase membrane Na permeability could be therapeutically useful.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabantchik Z. I., Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972 Dec 29;10(3):311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- Charache S., Walker W. G. Failure of desmopressin to lower serum sodium or prevent crisis in patients with sickle cell anemia. Blood. 1981 Nov;58(5):892–896. [PubMed] [Google Scholar]
- Clark M. R., Guatelli J. C., White A. T., Shohet S. B. Study on the dehydrating effect of the red cell Na+/K+-pump in nystatin-treated cells with varying Na+ and water contents. Biochim Biophys Acta. 1981 Sep 7;646(3):422–432. doi: 10.1016/0005-2736(81)90311-4. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Mohandas N., Shohet S. B. Deformability of oxygenated irreversibly sickled cells. J Clin Invest. 1980 Jan;65(1):189–196. doi: 10.1172/JCI109650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. R., Unger R. C., Shohet S. B. Monovalent cation composition and ATP and lipid content of irreversibly sickled cells. Blood. 1978 Jun;51(6):1169–1178. [PubMed] [Google Scholar]
- Dean J., Schechter A. N. Sickle-cell anemia: molecular and cellular bases of therapeutic approaches (third of three parts). N Engl J Med. 1978 Oct 19;299(16):863–870. doi: 10.1056/NEJM197810192991605. [DOI] [PubMed] [Google Scholar]
- Fahim M., Pressman B. C. Cardiovascular effects and pharmacokinetics of the carboxylic ionophore monensin in dogs and rabbits. Life Sci. 1981 Nov 9;29(19):1959–1966. doi: 10.1016/0024-3205(81)90604-4. [DOI] [PubMed] [Google Scholar]
- Glader B. E., Lux S. E., Muller-Soyano A., Platt O. S., Propper R. D., Nathan D. G. Energy reserve and cation composition of irreversibly sickled cells in vivo. Br J Haematol. 1978 Dec;40(4):527–532. doi: 10.1111/j.1365-2141.1978.tb05828.x. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Ross P. D., Eaton W. A. Kinetics and mechanism of deoxyhemoglobin S gelation: a new approach to understanding sickle cell disease. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4864–4868. doi: 10.1073/pnas.71.12.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary M., Abramson N. Induced hyponatremia for sickle-cell anemia. N Engl J Med. 1981 Apr 2;304(14):844–845. doi: 10.1056/NEJM198104023041414. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Jacobs M. S., Shohet S. B. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 1980 Sep;66(3):563–573. doi: 10.1172/JCI109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi C. T., Schechter A. N. The intracellular polymerization of sickle hemoglobin and its relevance to sickle cell disease. Blood. 1981 Dec;58(6):1057–1068. [PubMed] [Google Scholar]
- Orringer E. P., Roer M. E., Parker J. C. Cell density profile as a measure of erythrocyte hydration: therapeutic alteration of salt and water content in normal and SS red blood cells. Blood Cells. 1980;6(3):345–353. [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Rosa R. M., Bierer B. E., Thomas R., Stoff J. S., Kruskall M., Robinson S., Bunn H. F., Epstein F. H. A study of induced hyponatremia in the prevention and treatment of sickle-cell crisis. N Engl J Med. 1980 Nov 13;303(20):1138–1143. doi: 10.1056/NEJM198011133032002. [DOI] [PubMed] [Google Scholar]
- Scarpa A., Cecchetto A., Azzone G. F. The mechanism of anion translocation and pH equilibration in erythrocytes. Biochim Biophys Acta. 1970;219(1):179–188. doi: 10.1016/0005-2736(70)90073-8. [DOI] [PubMed] [Google Scholar]
- Serjeant G. R., Serjeant B. E., Desai P., Mason K. P., Sewell A., England J. M. The determinants of irreversibly sickled cells in homozygous sickle cell disease. Br J Haematol. 1978 Nov;40(3):431–438. doi: 10.1111/j.1365-2141.1978.tb05814.x. [DOI] [PubMed] [Google Scholar]


