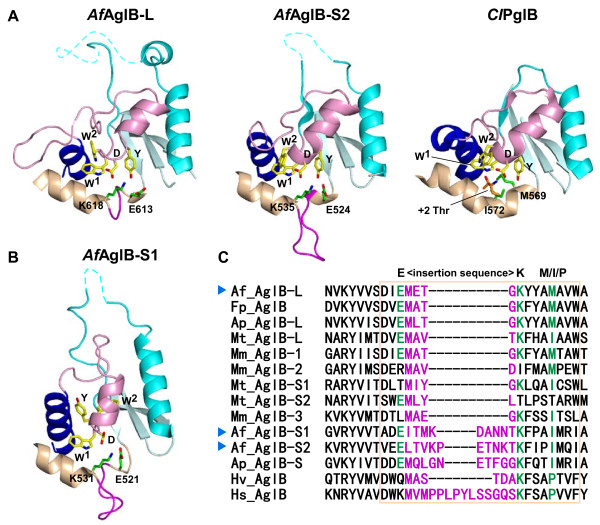
Figure 4.
Structural comparison of the Ser/Thr-binding pockets. (A) Close-up views of the Ser/Thr-binding pockets of A. fulgidus AglB-L and AglB-S2. The Ser/Thr pocket of C. lari PglB in the complex with the Thr residue in the glycosylation sequon is shown for comparison. The conformations of the Ser/Thr pockets are similar to each other, and represent the resting state and the peptide substrate-bound state. The same color code is used as in Figure 1. (B) Close-up view of the Ser/Thr-binding pocket of A. fulgidus AglB-S1. The structure of the Ser/Thr-binding pocket is distorted, due crystal packing effects or the lack of interactions with the TM region. (C) Multiple sequence alignment of the variant types of DK motifs found in the classes Halobacteria, Archaeoglobi, and Methanomicrobia. The alignment was performed with the program MAFFT, without manual adjustment. The three signature residues of the variant types of DK motifs are highlighted in green. The inserted loop sequences are shown in magenta. The region of the characteristic, kinked helices is enclosed in the light-brown box. The following AglB sequences were used: Af_AglB-L [UniProt/TrEMBL: O29867_ARCFU], Af_AglB-S1 [029918_ARCFU], Af_AglB-S2 [O30195_ARCFU], Ap_AglB-L [D2RHL5_ARCPA], Ap_AglB-S [D2RDQ2_ARCPA], Fp_AglB [D3S254_FERPA], Mm_AglB-1 [Q8PZ47_METMA], Mm_AglB-2 [Q8PZ48_METMA], Mm_AglB-3 [Q8PUW8_METMA], Mt_AglB-L [A0B996_METTP], Mt_AglB-S1 [A0B8C2_METTP], Mt_AglB-S2 [A0B9E5_METTP], Hv_AglB [A9JPF0_HALVO], and Hs_AglB [Q9HQP2_HALSA]. The sequences of the three paralogous AglB proteins from Archaeoglobus fulgidus are marked with blue arrowheads.