Abstract
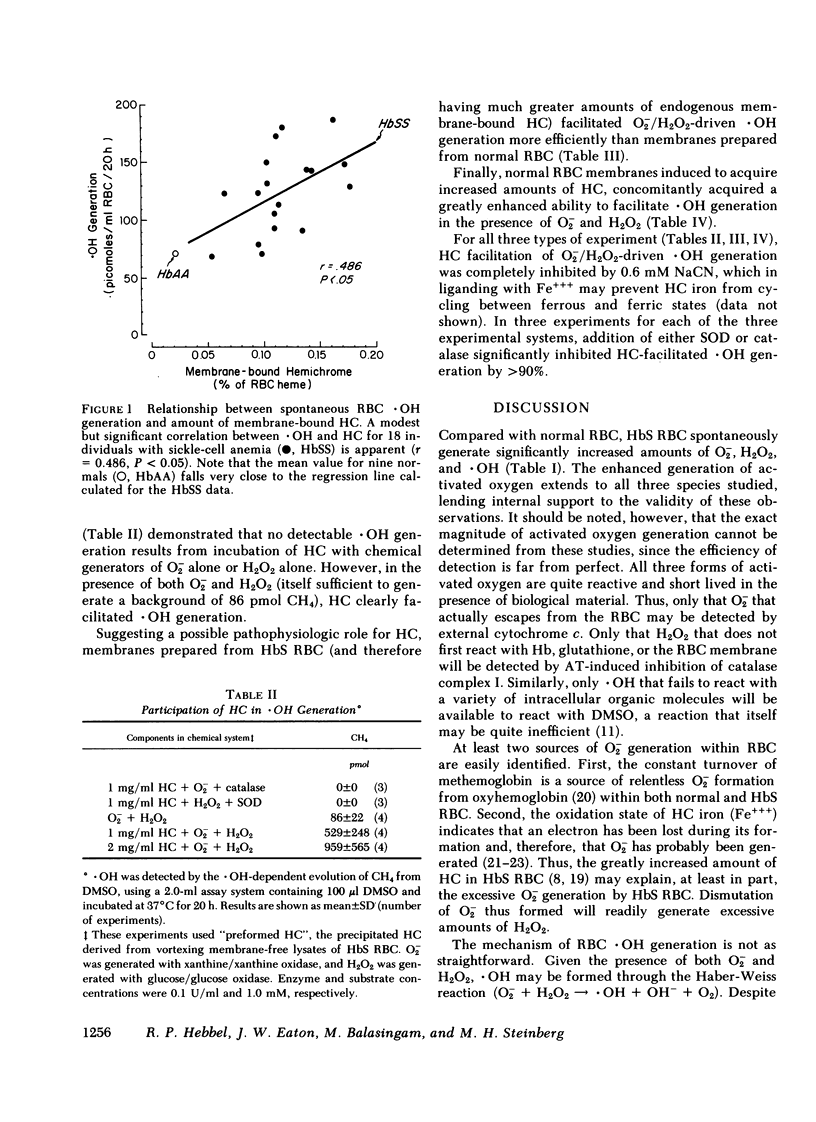
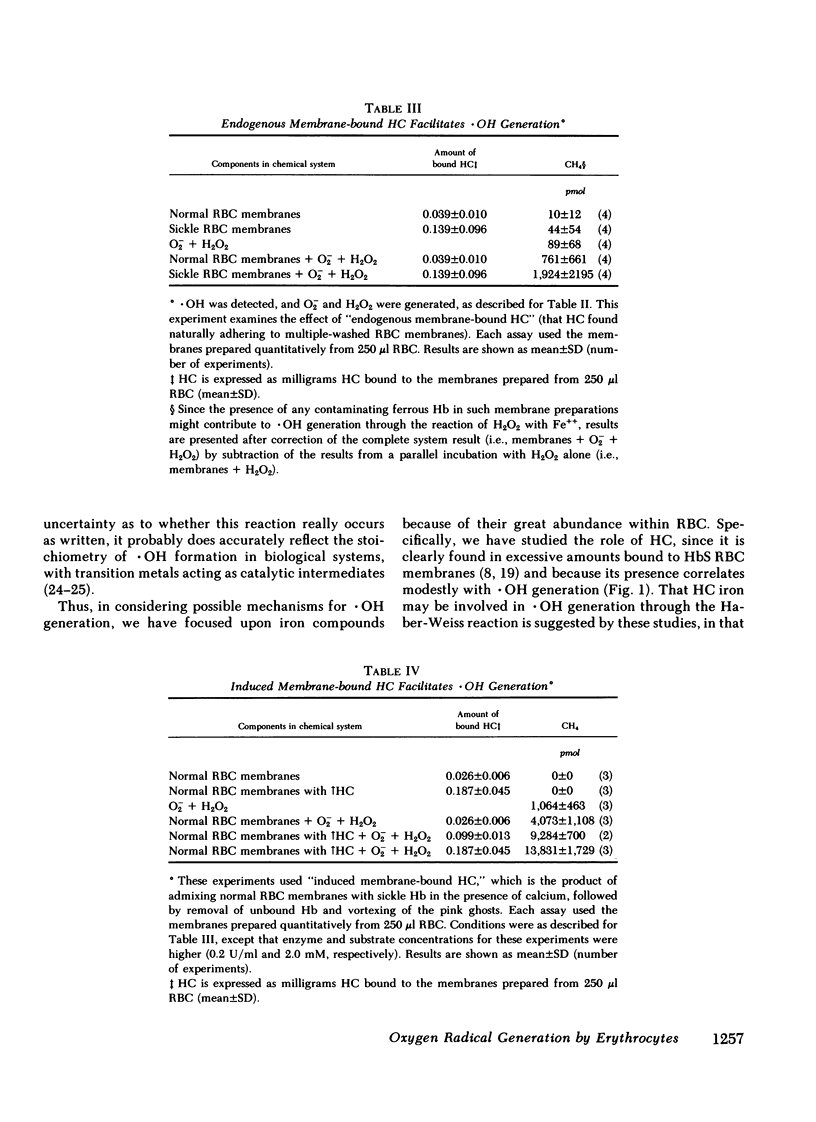
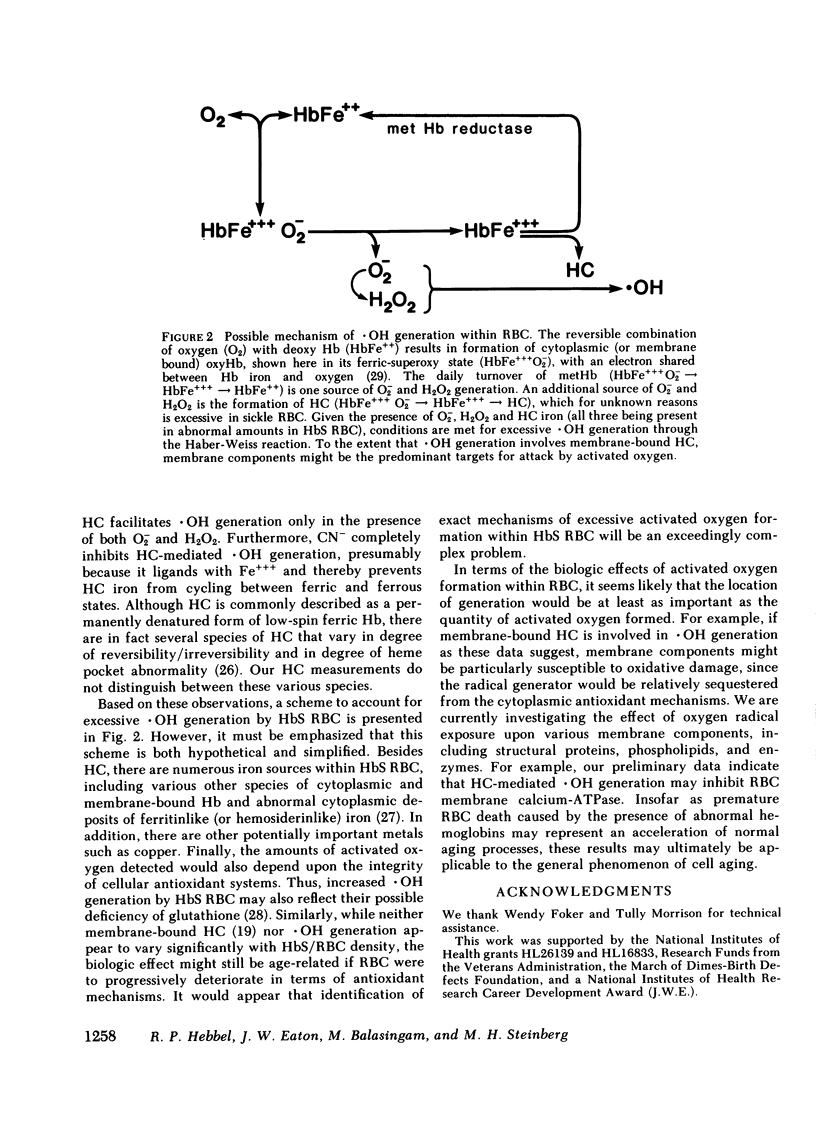
Since the various membrane abnormalities of sickle erythrocytes might result from excessive accumulation of oxidant damage, we have measured the generation of superoxide, peroxide, and hydroxyl radical by normal and sickle erythrocytes using assays involving reduction of cytochrome c, aminotriazole inhibition of catalase, and methane evolution from dimethyl sulfoxide, respectively. Compared with normal erythrocytes, sickle erythrocytes spontaneously generate approximately twice as much superoxide, peroxide, and hydroxyl radical. One possible source of hydroxyl radical generation was identified as hemichrome, excessive amounts of which are bound to sickle erythrocyte membranes. Hemichrome did not generate hydroxyl radical when exposed to superoxide alone or peroxide alone. However, in the presence of both superoxide and peroxide, hemichrome greatly facilitated hydroxyl radical generation. Supporting this, normal erythrocyte membranes induced to acquire sickle hemichrome concomitantly acquired an enhanced ability to mediate hydroxyl radical generation. Finally, sickle erythrocyte membranes greatly enhanced superoxide/peroxide-driven hydroxyl radical generation as compared with normal erythrocyte membranes. These data suggest that an excessive accumulation of oxidant damage in sickle erythrocyte membranes might contribute to the accelerated membrane senescence of these cells. They further indicate that accumulation of oxidant damage could be a determinant of normal erythrocyte membrane senescence.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakura T., Minakata K., Adachi K., Russell M. O., Schwartz E. Denatured hemoglobin in sickle erythrocytes. J Clin Invest. 1977 Apr;59(4):633–640. doi: 10.1172/JCI108681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauminger E. R., Cohen S. G., Ofer S., Rachmilewitz E. A. Quantitative studies of ferritinlike iron in erythrocytes of thalassemia, sickle-cell anemia, and hemoglobin Hammersmith with Mössbauer spectroscopy. Proc Natl Acad Sci U S A. 1979 Feb;76(2):939–943. doi: 10.1073/pnas.76.2.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E., Johnson C., Powars D., West C. Prevalence of glucose-6-phosphate dehydrogenase deficiency in sickle-cell disease. N Engl J Med. 1974 Apr 11;290(15):826–828. doi: 10.1056/NEJM197404112901504. [DOI] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GENERATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES BY HEMOLYTIC AGENTS. Biochemistry. 1964 Jul;3:895–900. doi: 10.1021/bi00895a006. [DOI] [PubMed] [Google Scholar]
- Campwala H. Q., Desforges J. F. Membrane-bound hemichrome in density-separated cohorts of normal (AA) and sickled (SS) cells. J Lab Clin Med. 1982 Jan;99(1):25–28. [PubMed] [Google Scholar]
- Carrell R. W., Winterbourn C. C., Rachmilewitz E. A. Activated oxygen and haemolysis. Br J Haematol. 1975 Jul;30(3):259–264. doi: 10.1111/j.1365-2141.1975.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Chiu D., Lubin B. Abnormal vitamin E and glutathione peroxidase levels in sickle cell anemia: evidence for increased susceptibility to lipid peroxidation in vivo. J Lab Clin Med. 1979 Oct;94(4):542–548. [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Das S. K., Nair R. C. Superoxide dismutase, glutathione peroxidase, catalase and lipid peroxidation of normal and sickled erythrocytes. Br J Haematol. 1980 Jan;44(1):87–92. doi: 10.1111/j.1365-2141.1980.tb01186.x. [DOI] [PubMed] [Google Scholar]
- Dixon E., Winslow R. M. The interaction between (Ca2+ + Mg2+)-ATPase and the soluble activator (calmodulin) in erythrocytes containing haemoglobin S. Br J Haematol. 1981 Mar;47(3):391–397. doi: 10.1111/j.1365-2141.1981.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Branda R. F., Hadland C., Dreher K. Anion channel blockade: effects upon erythrocyte membrane calcium response. Am J Hematol. 1980;9(4):391–399. doi: 10.1002/ajh.2830090406. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Yamada O., Moldow C. F., Jacob H. S., White J. G., Eaton J. W. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease. J Clin Invest. 1980 Jan;65(1):154–160. doi: 10.1172/JCI109646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila R. E., Cabbat F. S., Cohen G. In vivo inhibition of superoxide dismutase in mice by diethyldithiocarbamate. J Biol Chem. 1976 Apr 10;251(7):2182–2185. [PubMed] [Google Scholar]
- Lubin B., Chiu D., Bastacky J., Roelofsen B., Van Deenen L. L. Abnormalities in membrane phospholipid organization in sickled erythrocytes. J Clin Invest. 1981 Jun;67(6):1643–1649. doi: 10.1172/JCI110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Karnovsky M. J. Irreversible deformation of the spectrin-actin lattice in irreversibly sickled cells. J Clin Invest. 1976 Oct;58(4):955–963. doi: 10.1172/JCI108549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972 Nov 10;247(21):6960–6962. [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E., Rachmilewitz E. A. The demonstration of ferrihemochrome intermediates in heinz body formation following the reduction of oxyhemoglobin A by acetylphenylhydrazone. Biochim Biophys Acta. 1975 Jun 26;393(2):404–418. doi: 10.1016/0005-2795(75)90069-0. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaklai N., Sharma V. S., Ranney H. M. Interaction of sickle cell hemoglobin with erythrocyte membranes. Proc Natl Acad Sci U S A. 1981 Jan;78(1):65–68. doi: 10.1073/pnas.78.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L., Weinstein R. S., Straus J. H., Wallach D. F. Inside-out red cell membrane vesicles: preparation and purification. Science. 1970 Apr 10;168(3928):255–257. doi: 10.1126/science.168.3928.255. [DOI] [PubMed] [Google Scholar]
- Steinberg M. H., Adams J. G., 3rd Laboratory Diagnosis of sickling hemoglobinopathies. South Med J. 1978 Apr;71(4):413–416. doi: 10.1097/00007611-197804000-00021. [DOI] [PubMed] [Google Scholar]
- Stocks J., Offerman E. L., Modell C. B., Dormandy T. L. The susceptibility to autoxidation of human red cell lipids in health and disease. Br J Haematol. 1972 Dec;23(6):713–724. doi: 10.1111/j.1365-2141.1972.tb03486.x. [DOI] [PubMed] [Google Scholar]
- Wittenberg J. B., Wittenberg B. A., Peisach J., Blumberg W. E. On the state of the iron and the nature of the ligand in oxyhemoglobin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1846–1853. doi: 10.1073/pnas.67.4.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]


