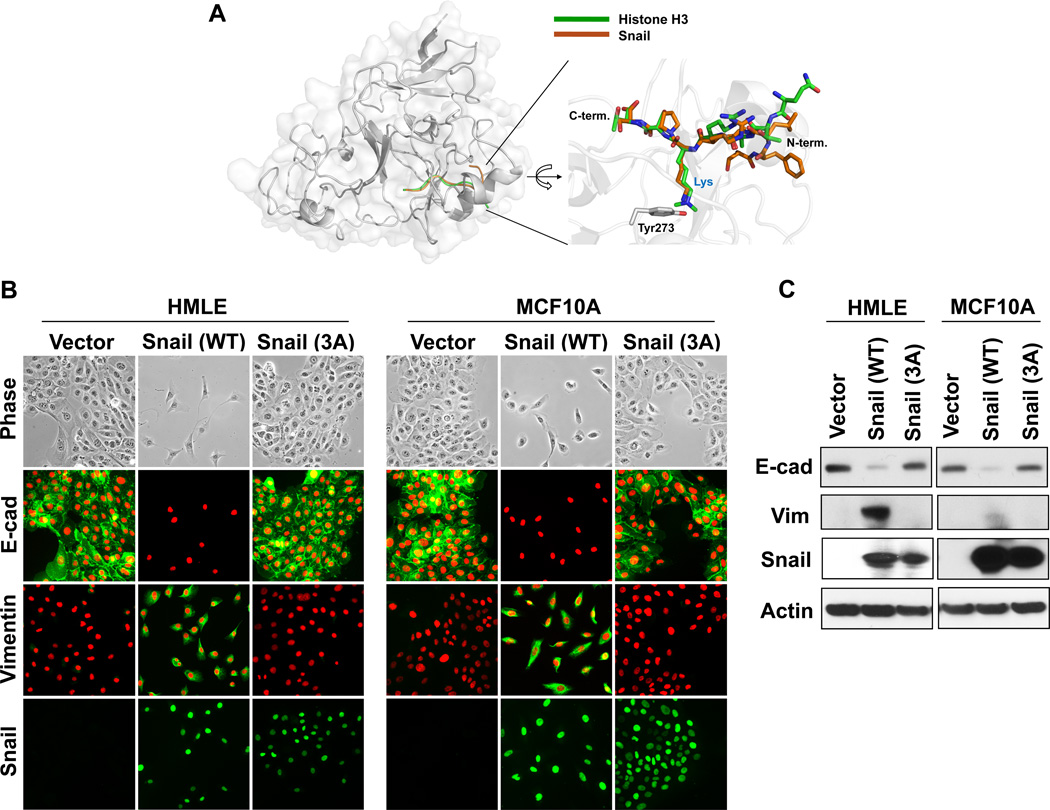
Figure 3.
Interaction of Snail with Suv39H1 is required for EMT induction. (A) A model structure of Suv39H1-Snail complex. The homolog (53% sequence identity) structure of GLP-Histone H3 peptide complex (PDB access code 2RFI) was used for the construction of this model (see Materials and Methods for details). The Snail and histone 3 peptides are shown in brown and green, respectively. A magnified view of the binding pocket is shown in the inset where the Suv39H1-Snail complex (brown) is superposed onto the GLP-histone H3 peptide complex structure (green). This ball-and-stick model highlights the position of the substrate lysine residue of Snail/H3 and the catalytic residue (Tyr273) of Suv39H1. In the GLP-histone H3 peptide complex structure, the lysine residue is modified as a form of N-dimethyl lysine to mimic the reaction product. (B) Wild-type and mutant Snail-3A (in which F5/K9/S11 were mutated to alanine) expressed in HMLE and MCF10A cells, the induction of EMT were analyzed by morphological changes and immunofluorescent staining of the expression of E-cadherin and vimentin. (C) Protein lysate from (B) were also examined for the expression of E-cadherin, vimentin and Snail by Western blotting.