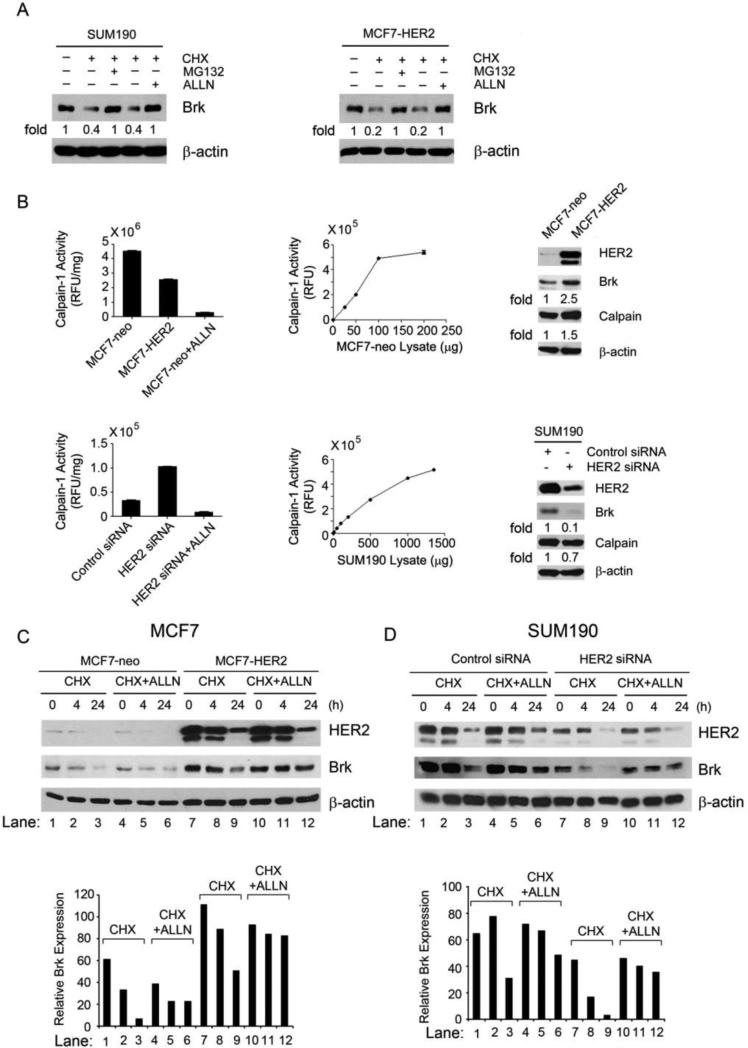
Fig. 3.
HER2-mediated inhibition of Brk proteolytic cleavage through the calpain-1 pathway. (A) SUM190 and MCF7-HER2 cells were untreated, treated with CHX (50 μg/mL) alone, or treated with CHX (50 μg/mL) plus proteasome inhibitor MG132 (5 μM) or calpain-1 inhibitor ALLN (20 μM) in 0.5% FBS medium as shown for 24 h. The cells were then lysed for Western blotting with the indicated antibodies. (B) Equal amounts (50 μg) of MCF7-neo and MCF7-HER2 cell lysates and equal amounts of control siRNA-treated and HER2 siRNA-treated SUM190 cell lysates (each in triplicate) were incubated with the fluorescently labeled calpain substrate II at 37°C for 30 min. Calpain activity was determined as described in Materials and Methods and plotted. The lysates of MCF7-neo cells and HER2 siRNA-treated SUM190 cells mixed with 25 μM ALLN were used as negative controls for calpain activity in vitro. Linear ranges of the enzymatic reaction with series of increasing amounts of MCF7-neo and SUM190 cell lysates are shown. The same lysates were also analyzed by Western blotting with the antibodies shown (right panels). (C, D) Pairs of MCF7-neo and MCF7-HER2 cells (C) and control siRNA-treated and HER2 siRNA-treated SUM190 cells (D) were untreated, treated with CHX (50 μg/mL) alone, or treated with CHX (50 μg/mL) plus ALLN (10 μM) for 4 h and 24 h, respectively. After the treatment, the cells were lysed for Western blotting with the indicated antibodies. Levels of Brk relative to the internal control β-actin were quantified and plotted as shown.