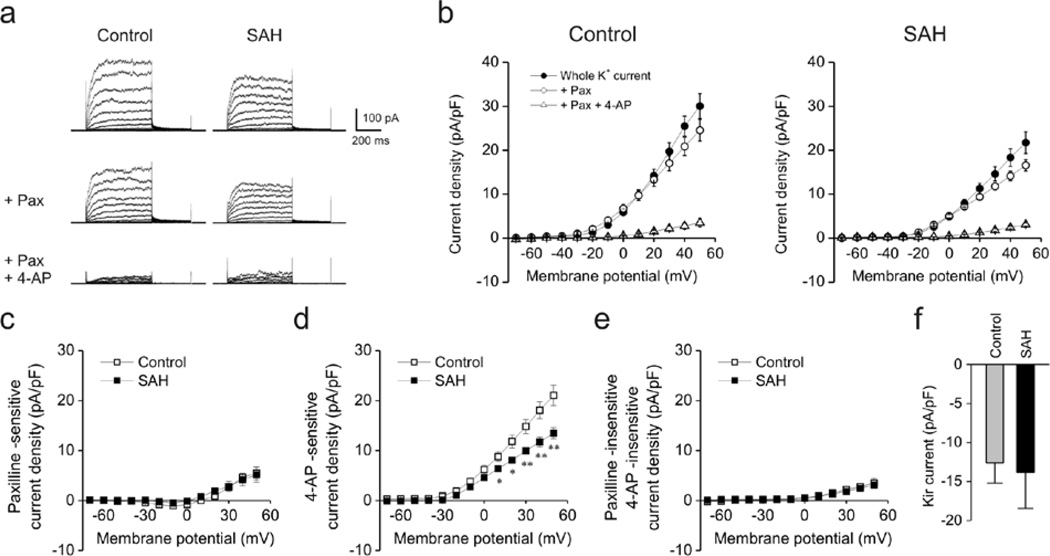
Figure 1. Decreased voltage-gated K+ (KV) currents in rabbit parenchymal arteriolar myocytes following SAH.
a) Representative traces of voltage-dependent K+ channel currents obtained using conventional whole cell patch clamp electrophysiology. Blockers of large conductance Ca2+-activated K+ (BK) channels (paxilline, Pax, 1 µM) and voltage-gated K+ (KV) channels (4-aminopyridine, 4-AP, 10 mM) were cumulatively added to cells at an interval of 10 min. Cell capacitance in control and SAH myoctes were 11.1 pF and 10.9 pF, respectively. b) Summary of current densities in the presence and absence of paxilline and 4-AP. Current density was calculated by dividing membrane currents by cell capacitance for each myocyte. Whole K+ currents (in the absence of paxilline and 4-AP, closed circles) were significantly smaller in parenchymal arteriolar myocytes from SAH model animals. Control: n = 6 cells from 2 animals, SAH: n = 5cells from 2 animals. c–e) Summary of K+ currents obtained in the presence of paxilline and 4-AP. 4-AP-sensitive (Kv) currents were decreased in myocytes obtained from SAH model animals (panel d). Paxilline-sensitive (panel c) and Paxilline-insensitive/4-AP-insensitive (panel e) currents were similar between groups. f) Inward-rectifier K+ (Kir) currents obtained at −100 mV were similar between Control (n = 15 cells from 8 animals) and SAH (n = 12 cells from 5 animals) myocytes.