Abstract
Rationale
We previously showed that early calcification of atherosclerotic plaques associates with macrophage accumulation. Chronic renal disease (CRD) and mineral imbalance accelerates calcification and the subsequent release of matrix vesicles (MVs) — precursors of microcalcification.
Objective
We tested the hypothesis that macrophage-derived MVs contribute directly to microcalcification.
Methods and Results
Macrophages associated with regions of calcified vesicular structures in human carotid plaques (n=136 patients). In vitro, macrophages released MVs with high calcification and aggregation potential. MVs expressed exosomal markers (CD9 and TSG101), and contained S100A9 and annexin V (Anx5). Silencing S100A9 in vitro and genetic deficiency in S100A9−/− mice reduced MV calcification, while stimulation with S100A9 increased calcification potential. Externalization of phosphatidylserine (PS) after Ca/P stimulation and interaction of S100A9 and Anx5, indicated that a PS-Anx5-S100A9 membrane complex facilitates hydroxyapatite nucleation within the macrophage-derived MV membrane.
Conclusions
Our results support the novel concept that macrophages release calcifying MVs enriched in S100A9 and Anx5, which contribute to accelerated microcalcification in CRD.
Keywords: Extracellular vesicles, macrophages, calcification, chronic renal disease, atherosclerosis, inflammation, plaque rupture
INTRODUCTION
Arterial microcalcification in the thin cap overlying the necrotic core in the intima of atherosclerotic plaques may cause compliance mismatch, increase local stress, and promote microfractures — leading to plaque rupture causing acute cardiovascular events1,2. Atherosclerotic plaques containing “spotty” calcifications consisting of calcified vesicles (precursors to microcalcification) have increased stress, thus making them more susceptible to rupture3.
Patients with chronic renal disease (CRD) have heightened susceptibility to atherosclerosis, a greater risk of intimal and medial calcification, and a high cardiovascular mortality4,5. The added complication of hyperphosphatemia may trigger release of matrix vesicles (MVs)6,7. Cells release MVs to initiate mineralization in both non-pathological8 and pathological conditions6–9. Smooth muscle cell (SMC)-derived MVs contribute to medial calcification in CRD patients7. Our molecular imaging studies co-localized early stages of atherosclerotic intimal calcification/microcalcification with macrophages10. During this initial phase of calcification, coincident inflammation and microcalcification suggest a direct role for macrophages in calcification11. In addition to the currently prevailing concept of osteogenic transition of SMCs, we hypothesized an alternative inflammation-related pathway of arterial calcification in which macrophages release microcalcification-generating MVs.
METHODS
Detailed Methods are available as an Online Supplement. Calcifying MVs were isolated from macrophages via ultracentrifugation7, and characterized comprehensively using histological, biochemical, and imaging techniques. To address the mechanisms for the mineralization of macrophage-derived MVs, we examined the contribution of S100A9 in gain-of-function and loss-of-function studies.
RESULTS
MVs released from macrophages contribute to intimal microcalcification
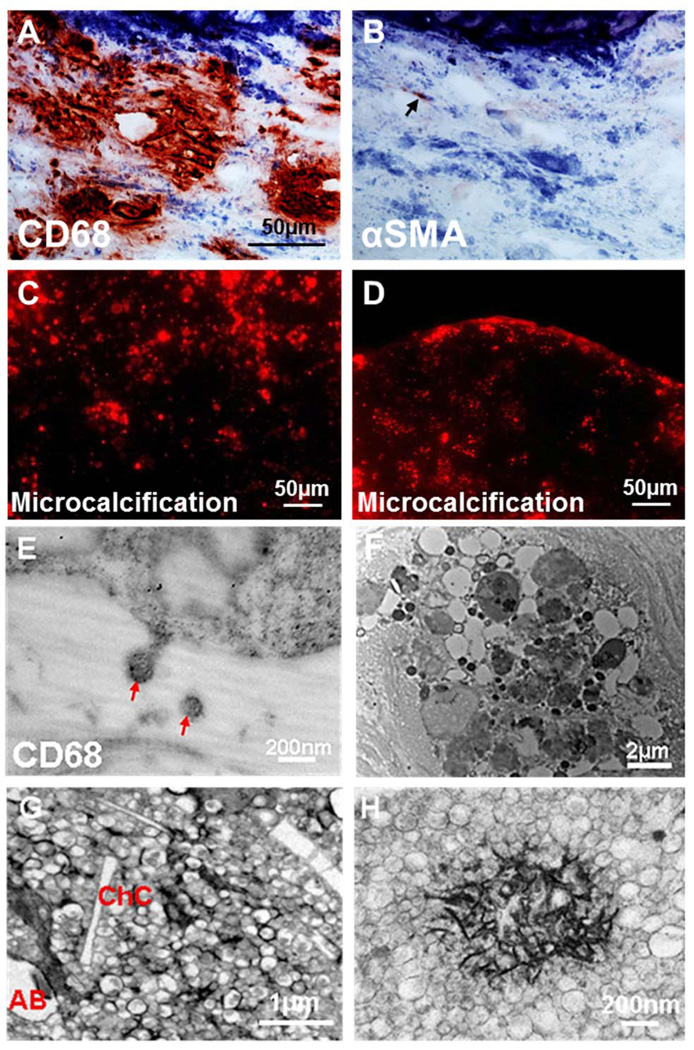
Immunohistochemical analysis of macrophages and SMCs (α-smooth muscle actin; α-SMA) in 136 human carotid endarterectomy samples and mouse arteries demonstrated that abundant macrophages associate with regions of MVs (Figure 1A), to a greater extent than α-SMA-positive or CD43-positive cells in areas of early spotty calcification, a characteristic associated with biomechanical instability (Figure 1B; Online Figures I–V). Ex vivo near-infrared fluorescence (NIRF) imaging of hydroxyapatite-binding nanoparticles visualized numerous calcifying MVs within calcified atherosclerotic plaques from humans (2784 ± 1073 MV/mm2; n=8; Figure 1C) and from apoE−/− mice (Figure 1D) with CRD, induced by 5/6 nephrectomy5. We used transmission electron microscopy (TEM) to characterize MVs within plaques. Immunogold labeling visualized release of CD68-positive MVs from macrophages (Figure 1E). MVs abound in human and mouse plaques, and associate with cholesterol crystals (Figures 1F and 1G). Plaque areas rich in MVs showed microcalcification and dense hydroxyapatite crystals localized on the membranes of calcifying MVs (Figure 1H).
Figure 1. Macrophage-derived MVs contribute to atherosclerotic microcalcification.
A, Calcified vesicular structures (hematoxylin; blue) adjacent to macrophages (CD68; red) in human plaques (n=127). B, Few to no αSMA-positive SMCs were noted in the same area. C, Ex vivo hydroxyapatite-binding NIRF imaging agent detected vesicular microcalcification (red) within human and, D, apoE−/− mouse plaques. E–F, Ultrastructure of human calcified plaques: E, Macrophage releases CD68-positive MVs (immunogold). F, Vesicles of different sizes and appearance. G–H, Ultrastructure of calcified CRD apoE−/− mouse plaque: G, MVs near cholesterol crystals (ChC; AB apoptotic bodies). H, MV (30–300 nm) hydroxyapatite crystallization.
Pro-inflammatory macrophages release microcalcification-generating MVs
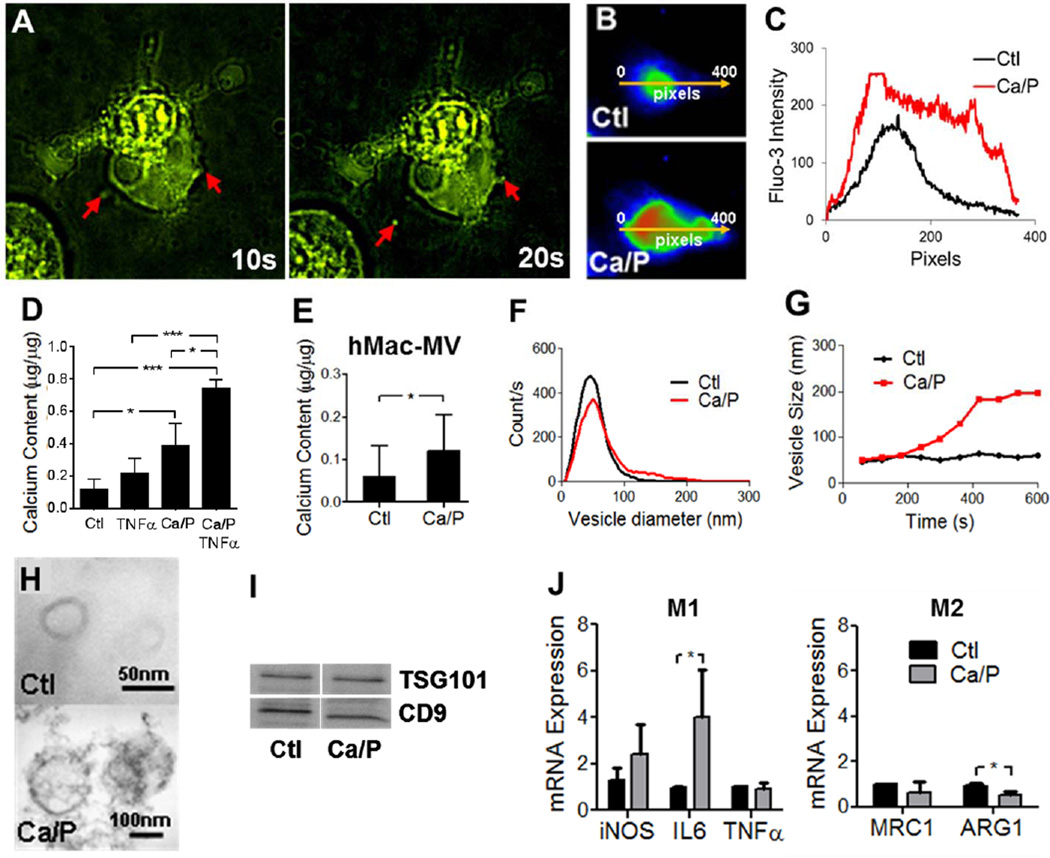
Time-lapse imaging of macrophages loaded with Fluo-3, a calcium indicator, showed vesicle release from membranous protrusions (Figure 2A; videos provided in Online Supplement) and calcium influx after Ca/P-stimulation (Figures 2B and 2C). Ca/P-stimulated macrophages released MVs (Mac-MVs) capable of mineralization, with increased calcium content (Figures 2D and 2E), alkaline phosphatase (ALP) activity (Online Figure VI), and suggested aggregation potential (Figures 2F and 2G). TEM of MVs released by control macrophages in vitro identified membrane-bound vesicles 30–300 nm, showing hydroxyapatite nucleation on the membrane and inside the vesicles after Ca/P stimulation (Figure 2H). Mac-MVs possessed exosomal markers (Figure 2I) and were smaller than those described for apoptotic bodies. Additionally, Ca/P stimulation did not affect the viability or apoptotic levels of mouse macrophages (Online Figure VII). Mouse macrophages stimulated with Ca/P possessed higher levels of mRNA encoding inducible nitric oxide synthase (iNOS) and interleukin-6 (IL-6) (Figure 2J), markers in mice of a pro-inflammatory “M1” macrophage polarization, while “M2” markers tended to decrease.
Figure 2. Macrophages release microcalcification-generating MVs.
A, Time-lapse imaging of mouse MV release from RAW264.7 (t = 0–20 seconds; video available in Online Supplement), B–C, The intensity of Fluo-3, a calcium indicator, within human macrophages (hMac) increased with Ca/Pstimulation, demonstrating calcium influx upon Ca/P stimulation. The arrow represents the x-axis on the graph. D–E, Various stimuli induce release of MVs with increased calcium content from mouse and human macrophages (n=3–7, *p<0.05, **p<0.001, ***p<0.0001; mean±SD). F–G, Mac-MVs from Ca/Pstimulated mouse macrophages were larger in size and potentially aggregated over time (n=3). H, Ultrastructure of mouse Mac-MVs released in vitro, showing membrane-bound MVs from control cells (top) and calcifying MVs (bottom). I, MVs expressed exosomal markers (TSG101 and CD9). J, Ca/Pstimulated mouse macrophages expressed pro-inflammatory markers (M1 phenotype) (n=3, *p<0.01 vs. control). Ca/P = 3 mmol/L calcium/2 mmol/L phosphate.
PS-S100A9-annexin V complex facilitates MV mineralization
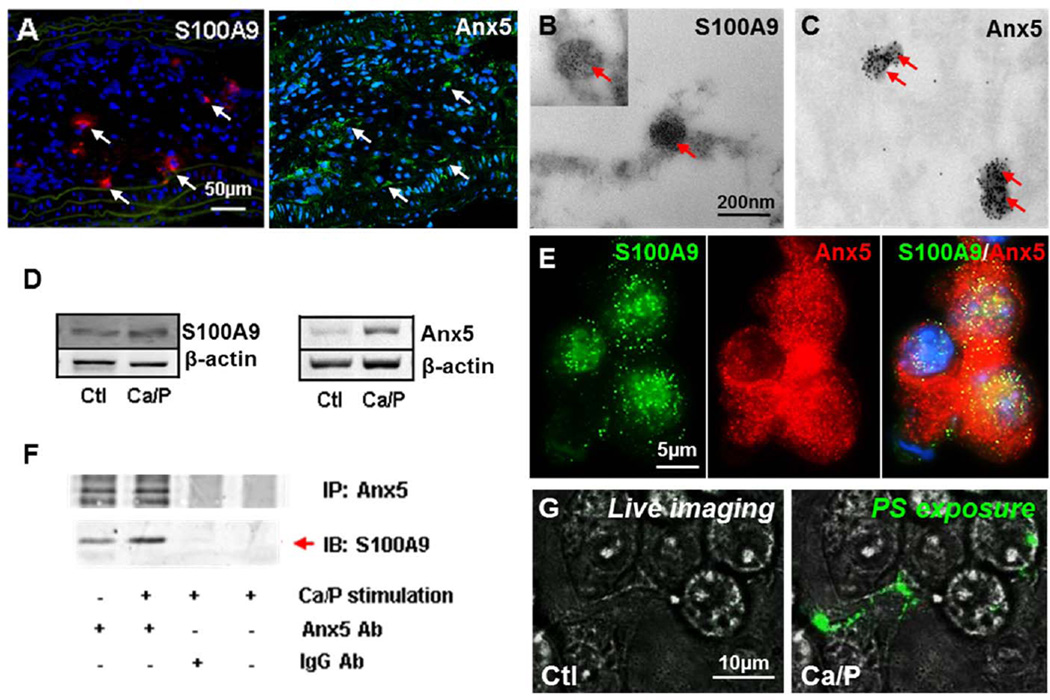
Annexins contribute to SMCs and osteoblast-derived MV-mediated calcification6,7,12. S100A9/MRP-14-positive MVs were identified in calcified plaques13. Calcified plaques of CRD apoE−/− mice showed expression of both S100A9 and Anx5 (Figure 3A; Online Figure VIII). In addition, S100A9 and Anx5 co-localized with MVs in calcified regions of human atheroma (immunogold TEM, Figures 3B and 3C). Ca/P stimulation enriched S100A9 and Anx5 expression in mouse Mac-MVs (Figure 3D). We hypothesized that S100A9 and Anx5 form a complex in Mac-MVs, similar to the complex formation of S100A9 and annexin VI14. The co-localization and co-immunoprecipitation analysis of S100A9 and Anx5 in cultured mouse macrophages supported this conjecture (Figures 3E and 3F). Ca/P stimulation of mouse macrophages augmented the association between S100A9 and Anx5 (Figure 3F). Anx5 and phosphatidylserine (PS) are critical for growth plate-MV mineral nucleation15, but whether nucleation occurs on the inside or outside of the vesicle is unclear. Ca/P-stimulated macrophages externalized PS, suggesting that nucleation may take place on the outer Mac-MV membrane (Figure 3G).
Figure 3. PS-Anx5-S100A9 complex facilitates MV mineralization.
A, Expression of S100A9 and Anx5 in calcified CRD apoE−/− mouse plaques (arrows). B, S100A9 and C, Anx5 co-localized with MVs in calcified human plaques (immunogold) (arrows point to MVs). D, Enriched S100A9 and Anx5 expression in calcifying mouse Mac-MVs (n=3). E, S100A9 (green) and annexin V (red) co-localized (yellow) in mouse macrophages; interaction confirmed by co-immunoprecipitation (F, n=3). G, PS externalization (green) noted with Ca/P stimulation.
S100A9 as a mediator of mineralization
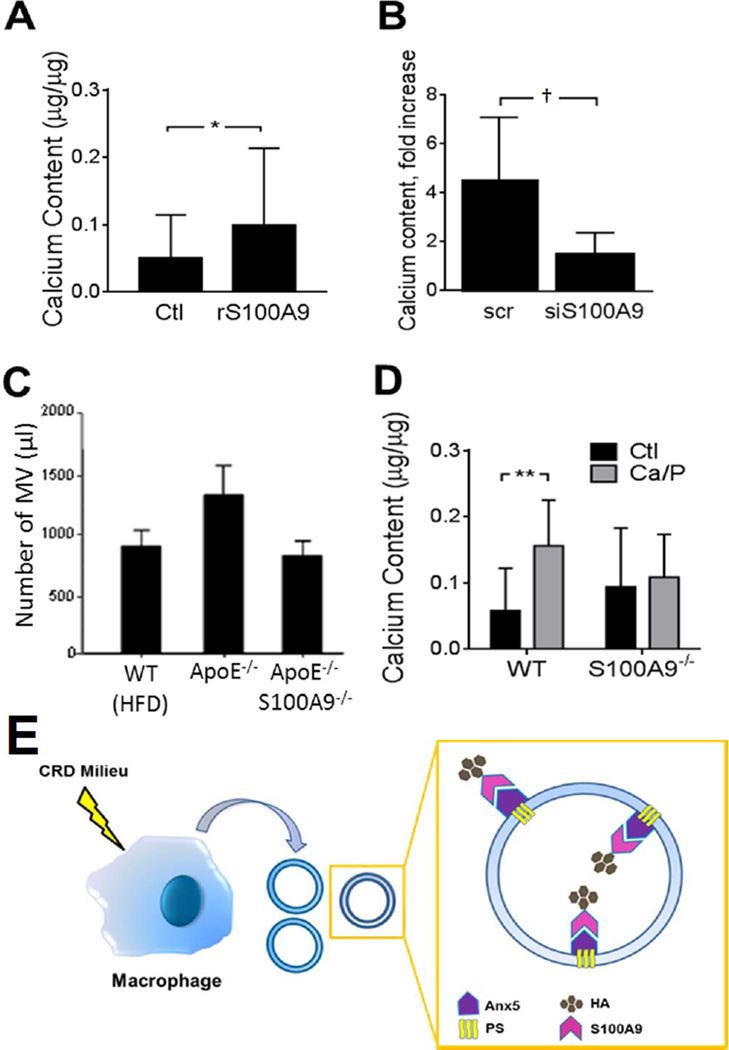
The addition of S100A9 in vitro enhanced the calcific potential of Mac-MVs (Figure 4A). S100A9 siRNA treatment in human macrophages (see Online Figure IX for silencing efficacy) limited the calcific potential of Mac-MVs (Figure 4B). To furnish further evidence in vivo for the function of S100A9 as a mediator of the calcific potential of Mac-MVs, we performed experiments using S100A9−/− apoE−/− mice. The level of MVs quantified in mouse plasma of fat-fed apoE−/− mice trended higher than in fat-fed wild-type (WT) mice, but did not achieve statistical significance with the number of animals studied. Inactivation of the S100A9 gene in apoE−/− mice tended to decrease MVs in plasma to the levels in WT mice (Figure 4C). In addition, Ca/P-stimulated S100A9−/− mouse peritoneal macrophages released Mac-MVs with lower calcific potential than those isolated from WT mice (Figure 4D).
Figure 4. S100A9 participates in MV calcification.
A, Addition of S100A9 enhanced the calcific potential of Mac-MVs released by mouse macrophages (n=11). [*p<0.05, **p<0.01, †p=0.057, mean±SD]. B, S100A9 siRNA transfection of human macrophages tended to decrease the calcific potential of MVs (n=4). C, MVs increased in apoE−/− mouse plasma (n=5) compared to WT mice (n=8). ApoE−/−S100A9−/− mice (n=5) had a lower MV concentration compared to apoE−/− mice (p=ns). D, Peritoneal macrophages from S100A9−/− mice tended to release MVs with a lower calcific potential, compared to WT mice (n=9). E, Proposed mechanism: Stimulated macrophages release MVs with a PSAnx5-S100A9 complex that facilitates the nucleation of hydroxyapatite and generation of microcalcification.
DISCUSSION
We report a novel pathway of microcalcification that involves macrophage-derived MVs. Concentrations of extracellular Ca and P, similar to those found in serum of CRD patients on dialysis, induced the release of Mac-MVs with higher calcification potential. These findings support the operation of a new additional mechanism of arterial calcification that likely accelerates the excessive intimal and medial calcification associated with CRD. Due to the relative abundance of macrophages over SMCs in rupture-prone plaques1, we hypothesize that macrophage-derived MVs participate in the initiation of microcalcification.
Mac-MVs were similar to previously reported chondrocyte-derived MVs (e.g., PS and Anx5 expression)12 and exosomes (CD9 and TSG101). Mineralization of growth plate chondrocytes by MVs requires PS and Anx5; indeed, other annexins may compensate for the loss of Anx515. This study found increased S100A9 and Anx5 expression in calcifying mac-MVs, highlighting their potential importance in MV calcification. The results provide evidence that S100A9 interacts with Anx5, forming a PS-Anx5-S100A9 membrane complex, which acts as a nucleation site for hydroxyapatite (Figure 4E).
The use of compound mutant mice, in addition to gain-of-function and loss-of-function in vitro experiments, permitted dissection of the role of S100A9 in the calcification of macrophage-derived MVs in vivo. Previous studies identified S100A9-positive macrophages in early atherosclerotic lesions and suggested the role of this calcium-binding protein in vascular inflammation16. The present evidence implicates S100A9 in the mineralization of macrophage-derived MVs.
This study provides new insight into the pathogenesis of vascular calcification, particularly microcalcification — a contributor to the acute thrombotic complications of atherosclerosis. Despite its clinical challenge, we currently lack treatments and diagnostic tools to prevent or reverse cardiovascular calcification. Molecular imaging provided a novel technique for visualizing calcifying vesicular structures that could be translated to the clinic. The evidence provided should help to develop therapies for this unmet medical need, including valvular as well as vascular calcification. Specifically, modulation of expression or function of S100A9 and/or Anx5 may suppress the nucleation of hydroxyapatite by macrophage-derived MVs and the generation of microcalcification in “spotty” plaques prone to rupture — thus preventing the onset of devastating clinical complications.
Supplementary Material
Novelty and Significance.
What Is Known?
Chronic renal disease (CRD) accelerates calcification in atherosclerotic plaques.
Microcalcification-rich plaques cause fatal ruptures.
Mineral imbalance induces release of vascular smooth muscle cell-derived matrix vesicles (MVs) – precursors of microcalcification.
What New Information Does This Article Contribute?
Macrophages directly contribute to the formation of microcalcification.
Pro-inflammatory macrophages release MVs capable of mineralization via formation of the phosphatidylserine-annexin 5-S100A9 membrane complex.
Disruption of MV formation may provide new therapeutic targets to combat calcific cardiovascular disease.
CRD patients are prone to medial and intimal calcification. The abundant macrophages in early microcalcification-rich atherosclerotic plaques suggest their importance in intimal calcification. Here, we demonstrate the direct contribution of macrophages to calcification via release of microcalcification-generating MVs, and provide new insight into their exosomal nature. These results reveal an alternative inflammation-related pathway of intimal calcification in atherosclerotic plaques, which is in contrast to the currently prevailing concept of osteogenic transition of smooth muscle cells. This understanding may open new therapeutic avenues to combat calcific arterial disease.
ACKNOWLEDGMENTS
We thank Christopher Duke, Chunyu Zheng, Eri Kamura, Dhruv Desai, Jacob Aaron, Eugenia Schvartz, Susan Hague, and Andrea Calhoun for their technical expertise, and Sara Karwacki for editorial assistance.
SOURCES OF FUNDING
This study was supported by grants from the National Institutes of Health (R01HL114805, to E.A.; K08HL086672-02, to K.C.; R01HL80472 to PL), and by a research grant from Kowa Company, Ltd.
Nonstandard Abbreviations
- Anx5
Annexin V
- Ca
Calcium
- CRD
Chronic renal disease
- HA
Hydroxyapatite
- hMac
Human macrophages
- Mac-MV
Macrophage-derived MVs
- MVs
Matrix vesicles
- NIRF
Near-infrared fluorescence
- P
Phosphate
- PS
Phosphatidylserine
- SD
Standard deviation
- siRNA
Small interfering RNA
- TEM
Transmission electron microscopy
- SMC
Smooth muscle cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med. 2002;8:1257–1262. doi: 10.1038/nm1102-1257. [DOI] [PubMed] [Google Scholar]
- 2.Li R, Mittelstein D, Lee J, Fang K, Majumdar R, Tintut Y, Demer LL, Hsiai TK. A dynamic model of calcific nodule destabilization in response to monocyte- and oxidized lipid-induced matrix metalloproteinases. Am J Physiol Cell Physiol. 2012;302:C658–C665. doi: 10.1152/ajpcell.00313.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenk JF, Papadopoulos P, Zohdi TI. Numerical modeling of stress in stenotic arteries with microcalcifications: a micromechanical approximation. J Biomech Eng. 2010;132 doi: 10.1115/1.4001351. 091011. [DOI] [PubMed] [Google Scholar]
- 4.Aikawa E, Aikawa M, Libby P, Figueiredo JL, Rusanescu G, Iwamoto Y, Fukuda D, Kohler RH, Shi GP, Jaffer FA, Weissleder R. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation. 2009;119:1785–1794. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman WG, London G, Amann K, Block GA, Giachelli C, Hruska KA, Ketteler M, Levin A, Massy Z, McCarron DA, Raggi P, Shanahan CM, Yorioka N. Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004;43:572–579. doi: 10.1053/j.ajkd.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Chen NX, O'Neill KD, Chen X, Moe SM. Annexin-mediated matrix vesicle calcification in vascular smooth muscle cells. J Bone Miner Res. 2008;23:1798–1805. doi: 10.1359/JBMR.080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapustin AN, Davies JD, Reynolds JL, McNair R, Jones GT, Sidibe A, Schurgers LJ, Skepper JN, Proudfoot D, Mayr M, Shanahan CM. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ Res. 2011;109:e1–e12. doi: 10.1161/CIRCRESAHA.110.238808. [DOI] [PubMed] [Google Scholar]
- 8.Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep. 2003;5:222–226. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- 9.Kim KM. Calcification of matrix vesicles in human aortic valve and aortic media. Fed Proc. 1976;35:156–162. [PubMed] [Google Scholar]
- 10.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 11.New SE, Aikawa E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ Res. 2011;108:1381–1391. doi: 10.1161/CIRCRESAHA.110.234146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirsch T, Wang W, Pfander D. Functional differences between growth plate apoptotic bodies and matrix vesicles. J Bone Miner Res. 2003;18:1872–1881. doi: 10.1359/jbmr.2003.18.10.1872. [DOI] [PubMed] [Google Scholar]
- 13.McCormick MM, Rahimi F, Bobryshev YV, Gaus K, Zreiqat H, Cai H, Lord RS, Geczy CL. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J Biol Chem. 2005;280:41521–41529. doi: 10.1074/jbc.M509442200. [DOI] [PubMed] [Google Scholar]
- 14.Bode G, Lüken A, Kerkhoff C, Roth J, Ludwig S, Nacken W. Interaction between S100A8/A9 and annexin A6 is involved in the calcium-induced cell surface exposition of S100A8/A9. J Biol Chem. 2008;283:31776–31784. doi: 10.1074/jbc.M803908200. [DOI] [PubMed] [Google Scholar]
- 15.Grskovic I, Kutsch A, Frie C, Groma G, Stermann J, Schlötzer-Schrehardt U, Nieholf A, Moss SE, Rosenbaum S, Pöschl E, Chmielewski M, Rappl G, Abken H, Bateman JF, Cheah KS, Paulsson M, Brachvogel B. Depletion of annexin A5, annexin A6 and collagen X causes no gross changes in matrix vesicle mediated mineralization, but lack of collagen X affects hematopoiesis and the Th1/Th2 response. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1682. online first. [DOI] [PubMed] [Google Scholar]
- 16.Croce K, Gao H, Wang Y, Mooroka T, Sakuma M, Shi C, Sukhova GK, Packard RR, Hogg N, Libby P, Simon DI. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation. 2009;120:427–436. doi: 10.1161/CIRCULATIONAHA.108.814582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.