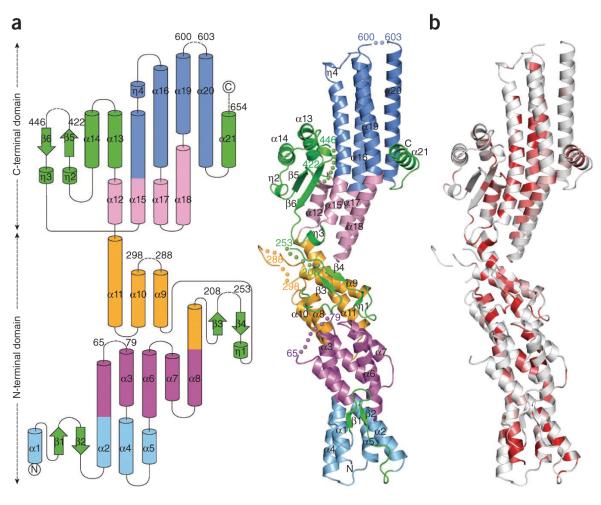
Figure 1.
The crystal structure of GCP4 reveals a previously undescribed fold. (a) Topology diagram (left) and ribbon representation (right). The first helix bundle is in light blue, the second in purple, the third in orange, the fourth in light pink and the fifth in blue. All structural elements excluded from helix bundles are in green. Helices and beta strands are numbered. Stretches of missing residues are represented by dashed lines (left) and by colored spheres (right). Residues preceding and following missing loops are labeled. (b) Ribbon representation colored according to sequence similarity over orthologous GCP4 proteins as shown in Supplementary Figure 1. Residues with similarity <80% are in white; conserved areas with similarity in the range 80–100% are colored light red to bright red.