Abstract
DNA damage triggers cell cycle arrest to provide a time window for DNA repair. Failure of arrest could lead to genomic instability and tumorigenesis. DNA damage-induced G1 arrest is generally achieved by the accumulation of Cyclin-dependent kinase inhibitor 1 (p21). However, p21 is degraded and does not play a role in UV-induced G1 arrest. The mechanism of UV-induced G1 arrest thus remains elusive. Here, we have identified a critical role for CUE domain-containing protein 2 (CUEDC2) in this process. CUEDC2 binds to and inhibits anaphase-promoting complex/cyclosome-Cdh1 (APC/CCdh1), a critical ubiquitin ligase in G1 phase, thereby stabilizing Cyclin A and promoting G1–S transition. In response to UV irradiation, CUEDC2 undergoes ERK1/2-dependent phosphorylation and ubiquitin-dependent degradation, leading to APC/CCdh1-mediated Cyclin A destruction, Cyclin-dependent kinase 2 inactivation, and G1 arrest. A nonphosphorylatable CUEDC2 mutant is resistant to UV-induced degradation. Expression of this stable mutant effectively overrides UV-induced G1–S block. These results establish CUEDC2 as an APC/CCdh1 inhibitor and indicate that regulated CUEDC2 degradation is critical for UV-induced G1 arrest.
DNA damage induced by various genotoxic stresses can jeopardize genomic integrity. UV light is the most pervasive environmental DNA-damaging agent, and accumulating evidence indicates that overexposure to UV light would increase the risk of skin cancer development. To maintain genomic stability, DNA damage response triggers cell cycle arrest, especially G1 arrest, which allows time for DNA repair and prevents aberrant replication of damaged DNA (1). Timely down-regulation of cell cycle promoters and rapid accumulation of cell cycle inhibitors are critical for DNA damage-induced G1 arrest. Earlier studies have indicated that the DNA damage-induced G1 arrest is mainly achieved by protein 53 (p53) activation and the subsequent p21 accumulation. However, Cyclin-dependent kinase inhibitor 1 (p21) is degraded following UV irradiation and does not play a role in this process (2). Thus, the molecular mechanism underlying UV-induced G1 arrest is not fully understood. Understanding the regulation of UV-induced G1 arrest will ultimately help to develop novel strategies for skin cancer prevention and therapy.
The anaphase-promoting complex or cyclosome (APC/C), a multisubunit E3 ubiquitin ligase, is an important regulator of protein degradation during the cell cycle. Activation of APC/C requires the association of either cell division cycle protein 20 (Cdc20) or Cdc20 homolog 1 (Cdh1), two related coactivators that recognize specific substrates containing the destruction box (D-box) or the lysine(K)-glutamic acid(E)-asparagine(N) (KEN) motif (3–5). Cdc20 functions in early mitosis, whereas Cdh1 has crucial functions in both late mitosis and G1 by targeting multiple cell cycle regulators, such as Cyclin A, Cyclin B1, and S-phase kinase-associated protein 2 (Skp2), for degradation (3, 4, 6–9). The destruction of Cyclin A and Skp2 prevents Cyclin-dependent kinase 2 (CDK2) activation and premature entry into S phase. To enter S phase, APC/CCdh1 must be turned off to allow for the reaccumulation of Cyclin A and Skp2 (10–12). However, how APC/CCdh1 is switched off is not fully understood. Recent studies have indicated that APC/CCdh1 is activated in response to DNA damage stress including UV irradiation and is crucial for maintaining genomic integrity (13–16). The underlying mechanism for APC/CCdh1 activation in DNA damage response also remains largely unknown.
CUE-domain-containing protein 2 (CUEDC2) plays critical roles in several important signaling pathways (17–21). Our recent work has demonstrated that CUEDC2 is phosphorylated by CDK1 and promotes spindle checkpoint inactivation through releasing APC/CCdc20 from checkpoint inhibition during mitosis (19). In the current study, we show that CUEDC2 exists in nonphosphorylated form in G1 phase, and inhibits APC/CCdh1 activity through binding to Cdh1 in a KEN-box–dependent manner. Upon UV treatment, ERK1/2 mediates CUEDC2 phosphorylation and triggers its degradation. Destruction of CUEDC2 releases APC/CCdh1 activity, resulting in Cyclin A destruction, CDK2 inactivation, and G1 arrest. A nonphosphorylatable stable CUEDC2 mutant overrides UV-induced G1 arrest. Collectively, our results identify CUEDC2 as a regulator of APC/CCdh1 and implicate its regulated degradation as an important mechanism for UV-induced G1 arrest.
Results
CUEDC2 Is Degraded During UV-Induced G1 Arrest and Its Overexpression Overcomes Such an Arrest.
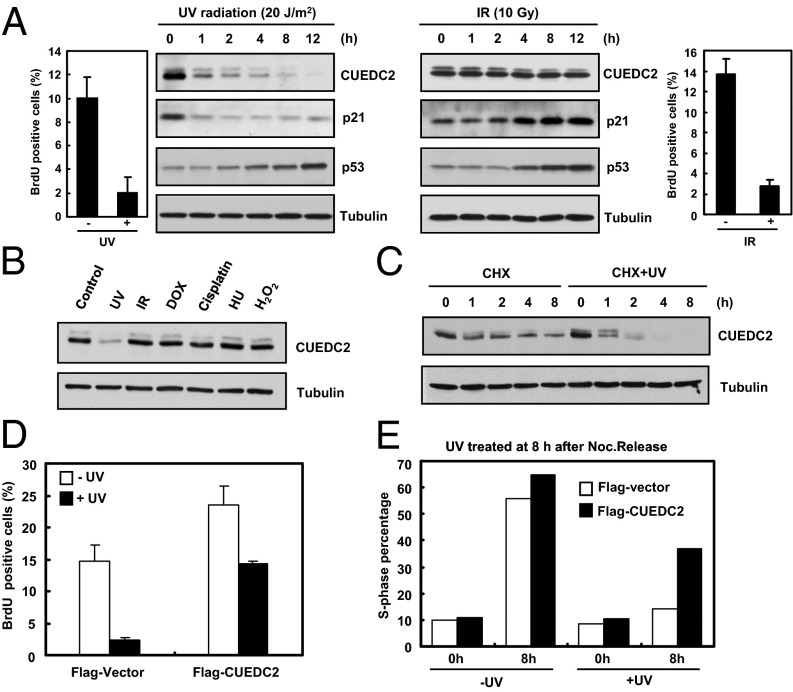
UV exposure is one of the main etiological causes of skin cancer. In a separate study, we found that CUEDC2 expression is significantly elevated in human skin cancer including melanoma and squamous cell carcinoma. We then explored the possible involvement of CUEDC2 in regulating DNA damage response following UV treatment. We first examined the protein levels of a variety of cell cycle regulators. As previously reported, p53 level is elevated while p21 is degraded after UV treatment (Fig. 1A, Left) (2). Interestingly, we found that CUEDC2 is rapidly down-regulated in various types of cells upon UV irradiation (Fig. 1A, Left, and Fig. S1 A–D). Surprisingly, CUEDC2 down-regulation is not triggered by other DNA-damaging agents, including IR irradiation (Fig. 1 A, Right, and B). To confirm that the reduction in CUEDC2 level was due to protein degradation, we treated the cells with cycloheximide and found that UV irradiation significantly shortened the half-life of CUEDC2 protein (Fig. 1C), suggesting that the UV-mediated destruction of CUEDC2 was caused by protein degradation. Because G1 arrest is a major consequence of UV irradiation, we next examined whether CUEDC2 might be involved in UV-induced G1 arrest. Strikingly, ectopic expression of CUEDC2 relieved the UV-induced G1 block in both asynchronous (Fig. 1D and Fig. S1E) and synchronized cells (Fig. 1E and Fig. S1F). These results suggested that CUEDC2 down-regulation is likely required for UV-induced G1 arrest.
Fig. 1.
CUEDC2 is degraded during UV-induced G1 arrest and its overexpression overcomes such an arrest. (A) Immunoblot analysis of CUEDC2 and other proteins in U2OS cells treated with UV-C (20 J/m2) irradiation or IR (10 Gy). The proportion of BrdU-positive cells were analyzed by FACS. (B) Detection of CUEDC2 protein levels in response to various DNA damage agents in U2OS cells. (C) U2OS cells were pretreated for 30 min with cycloheximide (20 mM) followed by UV treatment. CUEDC2 protein levels were determined as indicated. (D) MCF-10A stably expressing Flag-Vector or Flag-CUEDC2 cells were treated with or without UV-C (20 J/m2) irradiation. After an additional 4 h, cells were pulsed with BrdU (10 μM) for 1 h. The proportion of BrdU-positive cells were analyzed by FACS (error bars indicate SD; n = 3). (E) HeLa cells were transfected with Flag-Vector or Flag-CUEDC2, and 24 h later, cells were synchronized at mitosis by thymidine–nocodazole treatment, and then treated with or without UV-C (20 J/m2) irradiation at 8 h after nocodazole release and harvested at indicated times. Flag-Vector or Flag-CUEDC2–positive cells were analyzed for cell cycle distribution by FACS. The percentage of S-phase cells is shown in the histogram.
CUEDC2 Promotes G1–S Transition During the Normal Cell Cycle.
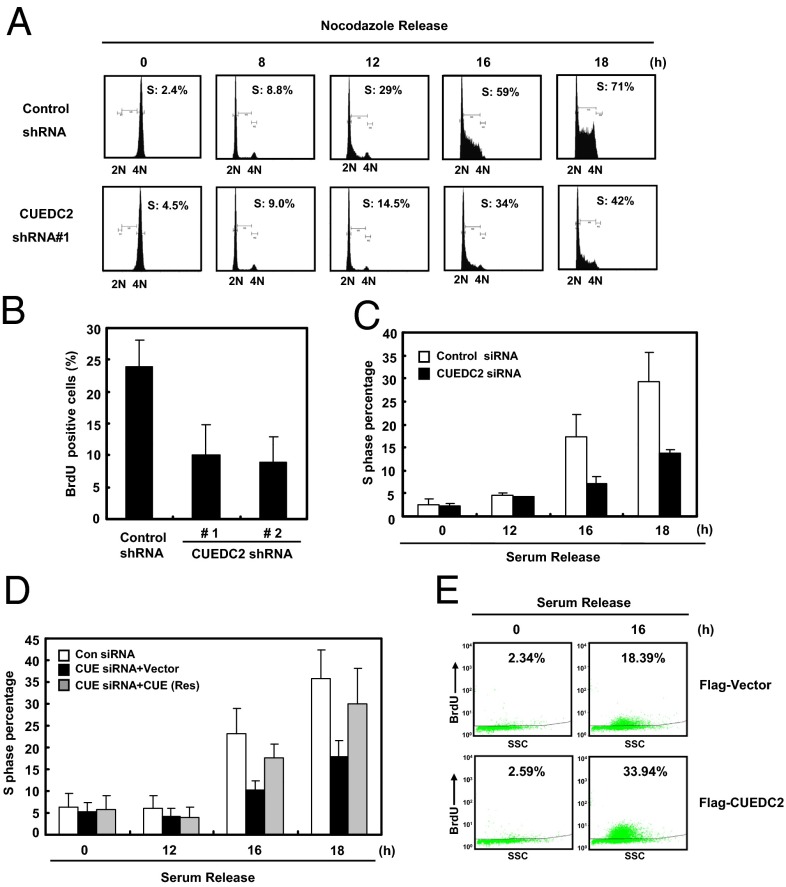
We next examined the physiological role of CUEDC2 in G1- to S-phase progression by knocking down CUEDC2. Results from FACS analysis of cells released from nocodazole arrest showed that the percentage of S-phase cells with CUEDC2 knockdown was much lower compared with the control cells (Fig. 2A). These results indicated that CUEDC2 knockdown indeed caused a marked delay in the cells entry into S phase, a similar effect as we observed in UV-exposed cells with CUEDC2 degradation. We further confirmed the finding by BrdU incorporation experiments with two different CUEDC2 shRNA (Fig. 2B and Fig. S2 A and B). In addition, we investigated the effect of CUEDC2 knockdown on the G1–S transition after cells were synchronized by serum starvation. Similarly as under nocodazole release condition, CUEDC2 knockdown dramatically reduced the percentage of S-phase cells following serum stimulation, indicating CUEDC2 specifically regulates G1–S transition (Fig. 2C and Fig. S2 C and D). Ectopic expression of RNAi-resistant CUEDC2 rescued this defect, ruling out the off-target effect of CUEDC2 RNAi (Fig. 2D and Fig. S2E). Consistently, overexpression of CUEDC2 promoted S-phase progression based on the BrdU incorporation assay (Fig. 2E and Fig. S2F). Taken together, these data suggested that CUEDC2 promotes G1-to-S transition.
Fig. 2.
CUEDC2 promotes G1–S transition during the normal cell cycle. (A) HeLa cells stably expressing control or CUEDC2 shRNA were synchronized at mitosis by thymidine–nocodazole treatment and released into fresh medium for the indicated times, and cell cycle distribution was monitored by FACS analysis. (B) HeLa cells stably expressing control or two different CUEDC2 shRNAs were synchronized at G1 phase by nocodazole releasing for 8 h, and then the cells were pulsed with BrdU (10 μM) for 2 h and fixed for immunofluorescence analysis. The proportion of BrdU-positive cells were shown, respectively (#1 and #2 are two different target sequences of CUEDC2 shRNAs). (C) MCF-10A cells were transfected with control or CUEDC2 siRNA and serum starved for 72 h, and then restimulated for the indicated time. The percentage of S-phase cells was analyzed by FACS (error bars indicate SD; n = 3). (D) MCF-10A cells with control or CUEDC2 knockdown and siRNA-resistant ectopic expression of CUEDC2, were treated as indicated. The percentage of S-phase cells were analyzed by FACS (error bars indicate SD; n = 3). (E) MCF-10A cells stably expressing Flag-Vector or Flag-CUEDC2 were pulsed with BrdU (10 μM) for 1 h at 16 h after serum release. The proportion of BrdU-positive cells was analyzed by FACS.
CUEDC2 Regulates Cyclin A Level and CDK2 Activity.
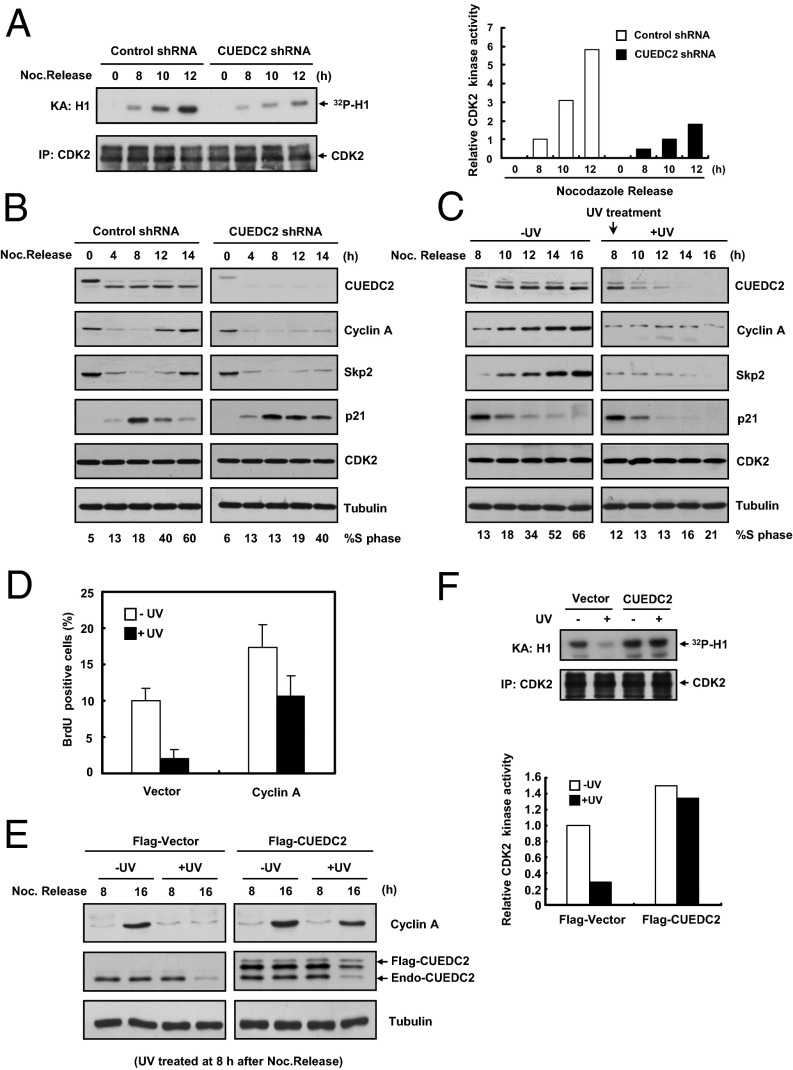
Because CDK2 is a key kinase for G1–S transition (22–25), we next investigated whether CUEDC2 has a role in the regulation of CDK2 activity. Compared with control cells, CUEDC2 knockdown clearly diminished the increase of CDK2 activity following nocodazole release (Fig. 3A). Conversely, overexpression of CUEDC2 resulted in an increase of CDK2 activity (Fig. S3A). To further investigate the mechanism by which CUEDC2 controls CDK2 activity, we examined the factors that are crucial in CDK2 activation during G1–S transition, such as Cyclin A and Skp2 (10, 22, 23, 26). As previously reported, Cyclin A and Skp2 were degraded during mitotic exit and reaccumulated in late G1 (Fig. 3B). CUEDC2 knockdown prevented the reaccumulation of Cyclin A and Skp2 following nocodazole release and delayed the S-phase entry (Fig. 3B). Consistently, p21 levels remained more stable in CUEDC2 knockdown cells (Fig. 3B), because Skp2 is its E3 ligase in G1 phase (26, 27). In addition, CUEDC2 overexpression increased the levels of Cyclin A and Skp2, but reduced p21 level (Fig. S3B). These results implied that CUEDC2 may regulate CDK2 activity through modulating Cyclin A and Skp2 levels.
Fig. 3.
CUEDC2 regulates Cyclin A level and CDK2 activity. (A) Kinase assays and immunoblotting of immunoprecipitated CDK2 following nocodazole release of HeLa cells with control or CUEDC2 shRNA. Quantification of band intensity is shown on the Right. (B) Immunoblot analysis of HeLa cells with control or CUEDC2 shRNA at indicated time points after nocodazole release. The percentage of S-phase cells was shown at Bottom, respectively. (C) Immunoassay of HeLa cells with or without UV-C (20 J/m2) irradiation at 8 h after nocodazole release and the indicated time points. The percentage of S-phase cells was shown at Bottom, respectively. (D) FACS analysis of HeLa cells transfected with control or Cyclin A expression vectors by BrdU incorporation assay (error bars indicate SD; n = 3). (E) Immunoassay of HeLa cells transfected with control or CUEDC2 expression vectors and treated or untreated by UV-C (20 J/m2) irradiation at 8 h after nocodazole release. (F) MCF-10A/Flag-Vector or MCF-10A/Flag-CUEDC2 were treated with or without UV-C (20 J/m2) irradiation, and 4 h after exposure, CDK2 activity was detected by in vitro kinase assay with Histone H1 as substrate; the relative corresponding quantity of CDK2 activity was shown by the bar chart at Bottom, respectively.
Next, we examined whether UV treatment had a similar effect on the levels of Cyclin A and Skp2. UV treatment resulted in an obvious G1–S block and a dramatic reduction of Cyclin A and Skp2 protein levels in cells released from nocodazole (Fig. 3C). As p21 is degraded following UV treatment and Skp2 regulates CDK2 activity by modulating p21 stability, it seems unlikely that the decrease of Skp2 plays a major role in UV-induced G1 arrest. However, because Cyclin A is a direct regulator of CDK2 activation, Cyclin A reduction might be critical for CDK2 inactivation in the UV-treated cells. Indeed, Cyclin A ectopic expression restored Cyclin A level in UV-treated cells (Fig. S3C) and overcame UV-induced G1 arrest based on BrdU incorporation assay (Fig. 3D and Fig. S3D). Importantly, CUEDC2 ectopic expression also partially restored Cyclin A protein level and CDK2 activity in UV-treated cells (Fig. 3 E and F). In contrast, CUEDC2 overexpression did not affect the reduction of Cdc25A protein level and accumulation of CDK2 phosphorylation (Thr14 and Tyr15), other mechanisms that were also involved in DNA damage-induced G1 arrest (2, 28) (Fig. S3E). These results suggested that CUEDC2 regulates UV-induced G1 arrest through Cyclin A destruction and CDK2 inactivation.
CUEDC2 Interacts with Cdh1 and Inhibits APC/CCdh1 Activity.
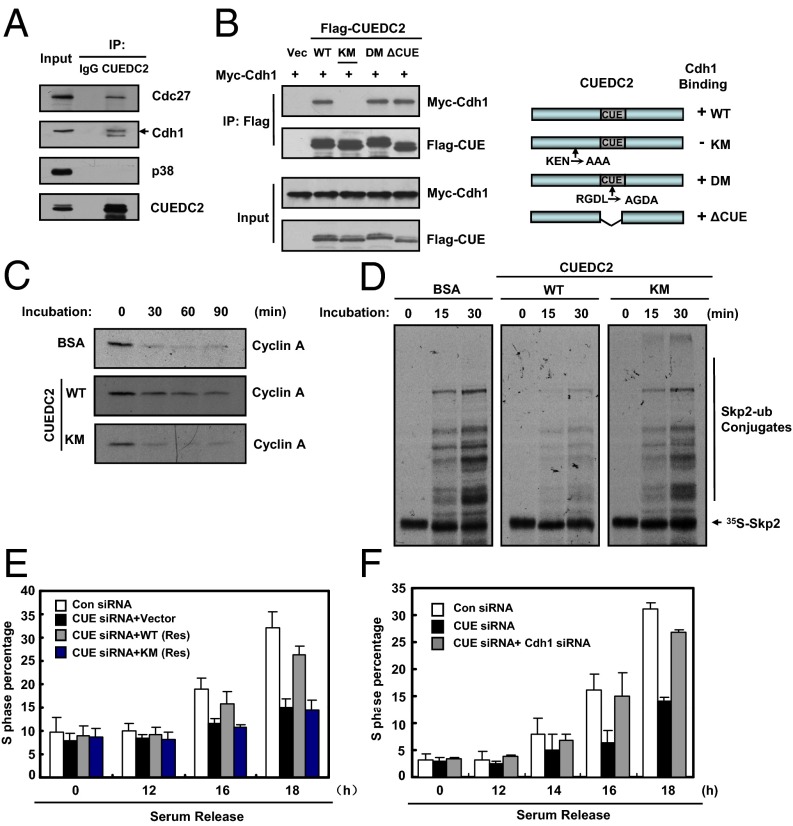
We then investigated how CUEDC2 regulates Cyclin A levels. The degradation of Cyclin A in G1 phase is mainly controlled by the E3 ligase complex of APC/CCdh1 (3, 4, 11, 29). Because CUEDC2 interacts with Cdc20 (19), we tested whether CUEDC2 also binds to its homolog, Cdh1. As shown in Fig. 4A, CUEDC2 indeed associated with Cdh1, and a core component of APC/C, Cdc27, in G1-phase cells. It is known that Cdh1-binding proteins usually have KEN box or D-box motifs (6, 7, 11). Because CUEDC2 contains both motifs, we tested whether these motifs mediated its interaction with Cdh1. We found that CUEDC2 WT, the D-box mutant (DM), and CUE domain mutant (ΔCUE) coimmunoprecipitated with Cdh1, whereas the KEN box mutant (KM) did not (Fig. 4B). The KEN box in APC/CCdh1 substrates generally binds to the propeller-shaped WD40 domain in Cdh1 (30, 31), we next mapped the region of Cdh1 binding to CUEDC2, and found that WD40 repeats of Cdh1 also mediated its interaction with CUEDC2 (Fig. S4 A and B). In addition, recombinant His-CUEDC2 WT, but not the KM mutant, interacted with Flag-Cdh1 obtained through in vitro translation (Fig. S4C). Thus, the KEN box of CUEDC2 is essential for its interaction with Cdh1. Either the CUE domain or the D-box is not required for the interaction.
Fig. 4.
CUEDC2 interacts with Cdh1 and inhibits APC/CCdh1 activity. (A) HeLa cells were synchronized at G1 phase at 4 h after nocodazole release. Cell lysates were immunoprecipitated with anti-CUEDC2 antibody or normal mouse IgG, and then the immunoprecipitates were analyzed by immunoblotting. (B) HEK293T cells were cotransfected with vectors expressing various Flag-tagged CUEDC2 and myelocytomatosis oncogene-tagged Cdh1. Cell lysates were subjected to immunoprecipitate with anti-Flag M2 beads. The immunoprecipitates and cell lysates were analyzed by immunoblotting. A schematic representation of the various CUEDC2 deletion mutants is shown at Right. KM, KEN-box mutant; DM, D-box mutant; ΔCUE, CUE domain deletion. (C) In vitro degradation assay by using Cyclin A as the substrate. HeLa cell extracts from G1 phase were preincubated with BSA or purified CUEDC2 proteins (WT or KM) for 30 min, and then supplemented with an energy-regenerating system, and reactions were initiated by the addition of Cyclin A protein. Samples were then harvested at the indicated times and analyzed by SDS/PAGE. (D) In vitro ubiquitination assay of HeLa cells treated and synchronized in G1 phase as described before. Cell extracts were incubated with in vitro-translated 35S-labeled Skp2 protein and purified CUEDC2 (WT) or CUEDC2 (KM) along with an energy-regenerating system at 30 °C for indicated times. Samples were detected by SDS/PAGE and autoradiography. (E) MCF-10A cells with control or CUEDC2 knockdown and siRNA-resistant ectopic expression of CUEDC2 (WT) or CUEDC2 (KM) were synchronized at G0/G1 by serum starvation, and then released into fresh medium for the indicated times, and S-phase percentage of cells was analyzed by FACS (error bars indicate SD; n = 3). (F) MCF-10A cells transfected with the indicated siRNA were synchronized at G0/G1 by serum starvation, and then released into fresh medium for the indicated times and S-phase percentage of cells was analyzed by FACS (error bars indicate SD; n = 3).
We next examined whether the binding of CUEDC2 to Cdh1 affected the APC/CCdh1 activity. We first used the in vitro Cyclin A degradation assay to test this possibility. Cyclin A was efficiently degraded in this system (Fig. 4C). Addition of CUEDC2 WT partially blocked Cyclin A degradation, whereas the KM mutant had no effect (Fig. 4C). Because the in vitro ubiquitination of Cyclin A by APC/CCdh1 was very inefficient, we chose to use another known APC/CCdh1 substrate, Skp2, to investigate CUEDC2 effect. The results showed that APC/CCdh1 activity was obviously inhibited by WT CUEDC2, but not the KEN-box mutant (Fig. 4D and Fig. S4D). These data suggested that CUEDC2 inhibits APC/CCdh1 activity through its KEN-box–dependent interaction with Cdh1. Taken together, CUEDC2 regulates Cyclin A level by inhibiting APC/CCdh1 activity at the G1–S transition.
As CUEDC2 could inhibit APC/CCdh1 activity, we next examined whether CUEDC2 regulates G1–S transition through Cdh1. First, we tested whether CUEDC2 KM mutant, which did not bind Cdh1 or inhibit APC/CCdh1 activity, could still promote G1–S transition. As shown above, the CUEDC2 WT rescued S-phase entry defect in CUEDC2 RNAi MCF-10A cells synchronized by serum starvation (Fig. 4E). By contrast, the KM mutant did not efficiently rescue this defect (Fig. 4E), suggesting that CUEDC2 might regulate G1–S transition through binding to Cdh1. To directly examine this possibility, we codepleted Cdh1 and CUEDC2 from MCF-10A cells and subjected them to serum starvation. CUEDC2 depletion alone delayed S-phase entry. Cdh1 knockdown mostly reversed the effect of CUEDC2 knockdown (Fig. 4F and Fig. S5A). Thus, the function of CUEDC2 on G1–S transition depends on Cdh1.
APC/CCdh1 has been shown to be involved in DNA damage response (14–16). We thus examined whether reduction of Cyclin A following UV treatment was mediated by APC/CCdh1. Cyclin A levels decreased in control RNAi cells after UV irradiation, but did not appreciably decrease in Cdh1 knockdown cells (Fig. S5B). The fact that Cyclin A reduction is partially blocked in Cdh1 knockdown cells suggested that APC/CCdh1 is responsible for UV-induced Cyclin A degradation.
Because the CUEDC2 KEN box binds to the WD40 domain of Cdh1, we investigated whether CUEDC2 served as a substrate of APC/CCdh1 in vivo. As shown in Fig. S5C, overexpression of Cdh1 resulted in a considerable decrease in the levels of Skp2, an APC/CCdh1 substrate. Surprisingly, CUEDC2 protein level was not affected by Cdh1 overexpression. Conversely, Cdh1 knockdown increased the levels of Skp2 and Cyclin A, but not that of CUEDC2 (Fig. S5D). Thus, CUEDC2 does not appear to be a substrate of APC/CCdh1. A similar finding has been observed for mitotic checkpoint serine/threonine-protein kinase BUB1 related protein (BubR1), which also contains KEN boxes but is not an efficient APC/C substrate (32).
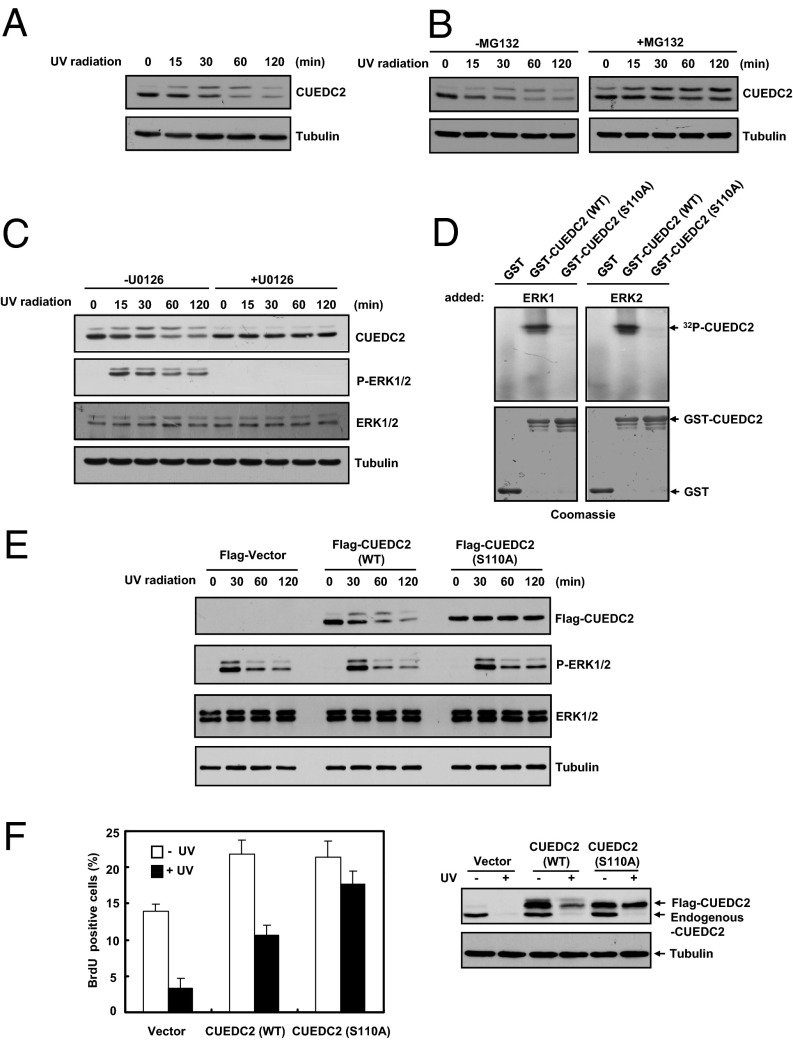
ERK1/2-Dependent Phosphorylation and Degradation of CUEDC2 Is Required for UV-Induced G1 Arrest.
As CUEDC2 is a positive regulator of G1-to-S phase progression and is degraded during UV-induced G1 arrest, we next explored the regulatory mechanism that triggered CUEDC2 degradation. Degradation of proteins is often preceded by their phosphorylation. We first detected whether CUEDC2 was phosphorylated at earlier time points before its degradation. Interestingly, we found that the upper band of CUEDC2 gradually accumulated before it was degraded after UV irradiation (Fig. 5A). Consistently, when we used specific proteasome inhibitor (MG132) to block CUEDC2 degradation, the lower band of CUEDC2 gradually decreased, whereas the upper band of CUEDC2 obviously increased (Fig. 5B). These results suggested that CUEDC2 might be phosphorylated before its degradation in response to UV. We have previously shown that CUEDC2 is phosphorylated by CDK1 in mitosis. However, CDK1 activity is inhibited upon UV treatment (33, 34), suggesting that CDK1 might not be responsible for UV-induced CUEDC2 phosphorylation. Further bioinformatics analysis suggested that CUEDC2 might be a potential substrate of ERK, which is known to be activated upon UV irradiation (35, 36). Indeed, the accumulation of CUEDC2 upper band upon UV irradiation was blocked by the addition of U0126, a well-established inhibitor of the ERK1/2-activating kinase MEK1/2 (Fig. 5C). These results suggested that CUEDC2 was phosphorylated by ERK1/2 following UV treatment. Notably, the UV-induced CUEDC2 degradation did not occur in the U0126-treated cells (Fig. 5C), suggesting that ERK1/2-mediated CUEDC2 phosphorylation is required for CUEDC2 degradation in response to UV.
Fig. 5.
ERK1/2-dependent phosphorylation and degradation of CUEDC2 is required for UV-induced G1 arrest. (A) Immunoblot analysis of CUEDC2 in MCF-10A cells treated with UV-C (20 J/m2) irradiation. (B) MCF-10A cells were pretreated with or without MG132 (10 μM) for 2 h before UV-C (20 J/m2) irradiation, and then CUEDC2 protein levels were detected at the indicated time. (C) MCF-10A cells were pretreated with or without U0126 (10 μM) for 30 min before UV-C (20 J/m2) irradiation. CUEDC2 and other proteins were detected as shown. (D) ERK1/2 kinase assays using GST vector, GST-CUEDC2 (WT), or CUEDC2 (S110A) as substrates. The amounts of recombinant proteins are shown below by Coomassie staining. (E) MCF-10A cells stably expressing Flag-Vector, Flag-CUEDC2 (WT), and Flag-CUEDC2 (S110A) were treated with UV-C (20 J/m2) irradiation. The CUEDC2 protein levels were detected at the indicated time. (F) MCF-10A cells stably expressing Flag-Vector, Flag-CUEDC2 (WT), and Flag-CUEDC2 (S110A) were treated and analyzed as in Fig. 1D. CUEDC2 protein levels were shown at Right.
CUEDC2 S110 has been previously identified as the major CDK1 target site; we checked whether S110 was also the ERK1/2 site. We performed an in vitro kinase assay and found that ERK1/ERK2 robustly phosphorylated the wild-type GST-CUEDC2, but not GST or GST-CUEDC2 S110A mutant (Fig. 5D), indicating that CUEDC2 is a substrate of ERK1/2 and is phosphorylated at S110. CUEDC2 S110A mutant expressed in MCF-10A cells does not undergo UV-induced gel mobility shift (Fig. 5E), suggesting that S110 is also a major ERK1/2 site in human cells.
To further confirm that CUEDC2 phosphorylation was required for UV-induced degradation, we examined UV-induced degradation of CUEDC2 WT and S110A. In response to UV treatment, CUEDC2 WT was gradually phosphorylated and degraded; however, CUEDC2 S110A, which could not be phosphorylated, was stable (Fig. 5E). Consistently, ubiquitination of CUEDC2 S110A was much weaker than CUEDC2 WT in response to UV irradiation (Fig. S6). These data further confirmed that phosphorylation of CUEDC2 at S110 is required for UV-induced CUEDC2 degradation. More important, expression of the nondegradable CUEDC2 S110A mutant was significantly more effective than CUEDC2 WT in overriding UV-induced G1–S block (Fig. 5F). These data indicated that the degradation of CUEDC2 is critical for UV-induced G1 arrest.
Early mitotic inhibitor 1 (Emi1) is a well-validated APC/CCdh1 inhibitor and overexpression of Emi1 accelerates S-phase entry (10), we next examined whether Emi1 was also eliminated as well as CUEDC2 upon UV treatment. However, we found that Emi1 level was not changed after UV irradiation, although CUEDC2 was degraded (Fig. S7A, Left). Geminin is a DNA replication licensing factor and an APC/CCdh1 substrate; its protein level was not down-regulated either (Fig. S7A, Left). In response to IR irradiation, neither of these proteins was degraded (Fig. S7A, Right). To rule out the possibility that the effect of CUEDC2 on Cyclin A level was due to its regulation on Emi1, we detect Emi1 protein level in CUEDC2 knockdown cells. Little change of Emi1 protein level was found either in asynchronized or in synchronized cells with CUEDC2 knockdown (Fig. S7B). Consistently, ectopic expression of CUEDC2, both its wild type and the nondegradable mutant, could not affect Emi1 level in response to UV treatment (Fig. S7C). These results indicated that CUEDC2 might be a direct regulator of APC/CCdh1. Because APC/CCdh1 is responsible for UV-induced Cyclin A degradation, we determined whether Emi1 overexpression could rescue the effect of UV irradiation on Cyclin A levels. As an APC/CCdh1 inhibitor, Emi1 ectopic expression could partially up-regulate Cyclin A levels in normal cells. However, ectopic expression of Emi1 could not rescue the effect of UV irradiation on Cyclin A levels (Fig. S7D). In response to UV irradiation, CUEDC2 is immediately degraded, which subsequently leads to APC/CCdh1-mediated Cyclin A degradation and G1 arrest. In contrast, the level of Emi1 is not changed following UV irradiation. Therefore, these data indicated that the degradation of CUEDC2 plays an important role in UV-induced G1/S arrest.
Discussion
The CDK inhibitor p21 protein is a main mediator of DNA damage-induced G1 arrest. Because p21 is degraded following UV treatment, it is puzzling how the UV-induced G1 arrest is achieved and what is the underlying molecular mechanism of this process. In this study, we show that CUEDC2 is a positive regulator for G1–S transition. Our results are consistent with the following model (Fig. S8). In this model, CUEDC2 binds to Cdh1 and inhibits APC/CCdh1 activity in a KEN-box–dependent manner, leading to the accumulation of Cyclin A and subsequent activation of CDK2, a key kinase for G1–S transition. When cells are exposed to UV light, CUEDC2 undergoes ERK1/2-mediated phosphorylation and degradation. Destruction of CUEDC2 releases APC/CCdh1 activity and promotes APC/CCdh1-mediated Cyclin A ubiquitination, leading to CDK2 inactivation and G1 arrest. These results further suggest that the UV-induced CUEDC2 degradation is an important step for cells to undergo G1 arrest and to prevent aberrant replication of damaged DNA. The dysregulation of CUEDC2 degradation in response to UV exposure might lead to genomic instability. Interestingly, in a separate study, we indeed found that CUEDC2 was highly expressed in various cancers, including melanoma and squamous cell carcinoma, indicating that CUEDC2 dysregulation might be involved in tumorigenesis.
APC/CCdh1 is responsible for the destruction of key cell cycle regulators, and its inactivation at the G1–S boundary allows the reaccumulation of Cyclins and subsequent CDK activation (29, 37). Several mechanisms have been shown to regulate APC/CCdh1, including Cdh1 phosphorylation, the degradation of its ubiquitin-conjugating enzyme E2 C (UBE2C/UbcH10), and the binding of Emi1 to Cdh1. In particular, Emi1 contains a D box and is believed to be a pseudosubstrate inhibitor of APC/C (10, 11, 38, 39). In our recent study on the role of CUEDC2 in mitosis, we have demonstrated that CUEDC2 is phosphorylated by CDK1, binds to the mitotic activator of APC/C, Cdc20, and mediates the release of APC/CCdc20 activity from Mad2 inhibition (19). In the current study, we have further shown that CUEDC2 binds to Cdh1, another APC/C activator in G1 phase, in a KEN-box–dependent manner. In contrast to the positive role of CUEDC2 in APC/CCdc20 activation, CUEDC2 appears to act as a pseudosubstrate inhibitor of APC/CCdh1, similar to Emi1. Thus, CUEDC2 promotes mitotic exit and G1–S transition through regulating APC/CCdc20 and APC/CCdh1, respectively.
Several lines of evidence suggest that APC/CCdh1 activation is required for DNA damage response (13, 15, 16). For example, maintaining APC/CCdh1 in an active state is essential for inhibiting G2/M transition in DNA damage response (13). In addition, APC/CCdh1-dependent proteolysis of ubiquitin-specific-processing protease 1 (Usp1) regulates the response to UV-induced DNA damage (14). However, the underlying molecular mechanisms of maintaining APC/CCdh1 in an active state after UV-induced DNA damage are still not clear. Our results indicate that UV triggers the degradation of CUEDC2, allowing the activation of APC/CCdh1. The enhanced APC/CCdh1 activity promotes the degradation of its substrate Cyclin A, resulting in G1 arrest. A nonphosphorylatable stable mutant of CUEDC2 overcomes UV-induced G1 arrest. CUEDC2 degradation is specifically induced by UV, but not other DNA damage agents. These results collectively indicate that CUEDC2 degradation-mediated release of APC/CCdh1 activity is a unique mechanism for UV-induced G1 arrest. Our findings provide an explanation for why UV-induced G1 arrest can still be achieved independent of p21, which is critical for G1 arrest triggered by other DNA damage agents but is degraded upon UV treatment. As we know, DNA damage signaling is majorly triggered by nuclear signal of damaged DNA. However, a separate study in our laboratory indicated that UV-induced destruction of CUEDC2 mainly occurs in cytoplasm and is most likely independent of DNA damage signaling. Interestingly, it has also been reported that NF-κB activation induced by UV does not depend on the nuclear signal (40). This finding raise the possibility that there may be some difference between UV and other DNA damage signaling, which might partially explain why only UV, not other DNA-damaging agents, leads to the rapid degradation of CUEDC2.
In conclusion, we have identified CUEDC2 as a pseudosubstrate inhibitor of APC/CCdh1. We further show that ERK1/2-regulated CUEDC2 degradation is required for UV-induced G1 arrest. Our study thus reveals a mechanism of G1 arrest following UV treatment and extends our knowledge on the regulation of G1–S transition.
Materials and Methods
Cell Cycle Analysis.
Cells were synchronized by thymidine–nocodazole arrest and shaken off. For G1-phase cells, nocodazole-arrested cells were released into fresh medium for 8 h. G0/G1-phase cells were obtained by serum starvation (0.2% serum) for 72 h. Cell cycle distributions were confirmed by flow cytometry. Thymidine–nocodazole arrest methods were performed as previously described (19, 41). For BrdU incorporation assay, UV-treated or untreated cells were pulsed with 10 μM BrdU (Sigma) for 1∼2 h at the indicated times. Ethanol (70%, vol/vol) and 4% (vol/vol) paraformaldehyde were used for fixing for FACS or immunofluorescence analysis, respectively. Cells were washed in PBS and incubated with 2 M HCl for 30 min. After rinsing in PBS-T, cells were incubated with BrdU antibody, FITC-conjugated rabbit anti-mouse IgG secondary antibody, and propidium iodide. A total of 10,000 or 400 cells was counted, respectively, under the flow cytometry or microscope and scored for positive BrdU staining.
In Vitro Ubiquitination and Degradation Assays.
These assays were performed as previously described (6, 9, 19, 38, 42). Briefly, G1-phase HeLa cell extracts were incubated with anti-Cdc27 antibody, and immune complexes were captured on protein G-Sepharose beads (GE Healthcare). After washing several times, beads were preincubated with purified CUEDC2 protein (WT, KM) or BSA for 30 min at 4 °C, and then incubated with 1 μL 35S-labeled Skp2 or purified Cyclin A protein with ubiquitination or degradation reaction systems. Aliquots were removed from 30 °C at indicated time, and resolved by SDS/PAGE, autoradiography, or Western blot.
Supplementary Material
Acknowledgments
We thank Dr. Marc W. Kirschner, Dr. Meloche Sylvai, Dr. Peter K. Jackson, and Dr. T. B. Kang for providing plasmids. This work was supported by National Basic Research Program of China Grants 2013CB910302, 2012CB910701, and 2012CB910801; International Science and Technology Cooperation Program of China Grant 2013DFA31710; Science Fund for Creative Research Groups Grant 81221004; National Key Technologies Research and Development Program for New Drugs Grant 2012ZX09301003-004; and National Natural Science Foundation of China Grants 31271447, 31100978, 81130037, and 81025010.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221009110/-/DCSupplemental.
References
- 1.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432(7015):316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 2.Bendjennat M, et al. UV irradiation triggers ubiquitin-dependent degradation of p21(WAF1) to promote DNA repair. Cell. 2003;114(5):599–610. doi: 10.1016/j.cell.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Visintin R, Prinz S, Amon A. CDC20 and CDH1: A family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278(5337):460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- 4.Fang G, Yu H, Kirschner MW. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell. 1998;2(2):163–171. doi: 10.1016/s1097-2765(00)80126-4. [DOI] [PubMed] [Google Scholar]
- 5.Herzog F, et al. Structure of the anaphase-promoting complex/cyclosome interacting with a mitotic checkpoint complex. Science. 2009;323(5920):1477–1481. doi: 10.1126/science.1163300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfleger CM, Kirschner MW. The KEN box: An APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14(6):655–665. [PMC free article] [PubMed] [Google Scholar]
- 7.Burton JL, Solomon MJ. D box and KEN box motifs in budding yeast Hsl1p are required for APC-mediated degradation and direct binding to Cdc20p and Cdh1p. Genes Dev. 2001;15(18):2381–2395. doi: 10.1101/gad.917901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428(6979):190–193. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- 9.Wei W, et al. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428(6979):194–198. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- 10.Hsu JY, Reimann JD, Sørensen CS, Lukas J, Jackson PK. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1) Nat Cell Biol. 2002;4(5):358–366. doi: 10.1038/ncb785. [DOI] [PubMed] [Google Scholar]
- 11.Miller JJ, et al. Emi1 stably binds and inhibits the anaphase-promoting complex/cyclosome as a pseudosubstrate inhibitor. Genes Dev. 2006;20(17):2410–2420. doi: 10.1101/gad.1454006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, et al. Deubiquitinase USP37 is activated by CDK2 to antagonize APC(CDH1) and promote S phase entry. Mol Cell. 2011;42(4):511–523. doi: 10.1016/j.molcel.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Bassermann F, et al. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell. 2008;134(2):256–267. doi: 10.1016/j.cell.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotto-Rios XM, Jones MJ, Busino L, Pagano M, Huang TT. APC/CCdh1-dependent proteolysis of USP1 regulates the response to UV-mediated DNA damage. J Cell Biol. 2011;194(2):177–186. doi: 10.1083/jcb.201101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudo T, et al. Activation of Cdh1-dependent APC is required for G1 cell cycle arrest and DNA damage-induced G2 checkpoint in vertebrate cells. EMBO J. 2001;20(22):6499–6508. doi: 10.1093/emboj/20.22.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T, Nirantar S, Lim HH, Sinha I, Surana U. DNA damage checkpoint maintains CDH1 in an active state to inhibit anaphase progression. Dev Cell. 2009;17(4):541–551. doi: 10.1016/j.devcel.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Zhang PJ, et al. CUE domain containing 2 regulates degradation of progesterone receptor by ubiquitin-proteasome. EMBO J. 2007;26(7):1831–1842. doi: 10.1038/sj.emboj.7601602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li HY, et al. Deactivation of the kinase IKK by CUEDC2 through recruitment of the phosphatase PP1. Nat Immunol. 2008;9(5):533–541. doi: 10.1038/ni.1600. [DOI] [PubMed] [Google Scholar]
- 19.Gao YF, et al. Cdk1-phosphorylated CUEDC2 promotes spindle checkpoint inactivation and chromosomal instability. Nat Cell Biol. 2011;13(8):924–933. doi: 10.1038/ncb2287. [DOI] [PubMed] [Google Scholar]
- 20.Pan X, et al. Elevated expression of CUEDC2 protein confers endocrine resistance in breast cancer. Nat Med. 2011;17(6):708–714. doi: 10.1038/nm.2369. [DOI] [PubMed] [Google Scholar]
- 21.Zhang WN, et al. CUEDC2 (CUE domain-containing 2) and SOCS3 (suppressors of cytokine signaling 3) cooperate to negatively regulate Janus kinase 1/signal transducers and activators of transcription 3 signaling. J Biol Chem. 2012;287(1):382–392. doi: 10.1074/jbc.M111.276832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: Does one fit all? Nat Rev Mol Cell Biol. 2008;9(11):910–916. doi: 10.1038/nrm2510. [DOI] [PubMed] [Google Scholar]
- 23.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: A changing paradigm. Nat Rev Cancer. 2009;9(3):153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 24.Yang ZF, Mott S, Rosmarin AG. The Ets transcription factor GABP is required for cell-cycle progression. Nat Cell Biol. 2007;9(3):339–346. doi: 10.1038/ncb1548. [DOI] [PubMed] [Google Scholar]
- 25.Rodier G, et al. p107 inhibits G1 to S phase progression by down-regulating expression of the F-box protein Skp2. J Cell Biol. 2005;168(1):55–66. doi: 10.1083/jcb.200404146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: Tipping the scales of cancer. Nat Rev Cancer. 2008;8(6):438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu G, Lozano G. p21 stability: Linking chaperones to a cell cycle checkpoint. Cancer Cell. 2005;7(2):113–114. doi: 10.1016/j.ccr.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Mailand N, et al. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288(5470):1425–1429. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- 29.Skaar JR, Pagano M. Cdh1: A master G0/G1 regulator. Nat Cell Biol. 2008;10(7):755–757. doi: 10.1038/ncb0708-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraft C, Vodermaier HC, Maurer-Stroh S, Eisenhaber F, Peters JM. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol Cell. 2005;18(5):543–553. doi: 10.1016/j.molcel.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 31.Jia L, Yu H. Cdh1 is a HECT of an activator. Mol Cell. 2011;44(5):681–683. doi: 10.1016/j.molcel.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Lara-Gonzalez P, Scott MI, Diez M, Sen O, Taylor SS. BubR1 blocks substrate recruitment to the APC/C in a KEN-box-dependent manner. J Cell Sci. 2011;124(Pt 24):4332–4345. doi: 10.1242/jcs.094763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boutros R, Dozier C, Ducommun B. The when and wheres of CDC25 phosphatases. Curr Opin Cell Biol. 2006;18(2):185–191. doi: 10.1016/j.ceb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Chen D, Vogel J. SAD kinase keeps centrosomes lonely. Nat Cell Biol. 2009;11(9):1047–1048. doi: 10.1038/ncb0909-1047. [DOI] [PubMed] [Google Scholar]
- 35.Moniz LS, Stambolic V. Nek10 mediates G2/M cell cycle arrest and MEK autoactivation in response to UV irradiation. Mol Cell Biol. 2011;31(1):30–42. doi: 10.1128/MCB.00648-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrlich P, Karin M, Weiss C. Supreme EnLIGHTenment: Damage recognition and signaling in the mammalian UV response. Mol Cell. 2008;29(3):279–290. doi: 10.1016/j.molcel.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wäsch R, Robbins JA, Cross FR. The emerging role of APC/CCdh1 in controlling differentiation, genomic stability and tumor suppression. Oncogene. 2010;29(1):1–10. doi: 10.1038/onc.2009.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rape M, Kirschner MW. Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry. Nature. 2004;432(7017):588–595. doi: 10.1038/nature03023. [DOI] [PubMed] [Google Scholar]
- 39.Sørensen CS, et al. A conserved cyclin-binding domain determines functional interplay between anaphase-promoting complex-Cdh1 and cyclin A-Cdk2 during cell cycle progression. Mol Cell Biol. 2001;21(11):3692–3703. doi: 10.1128/MCB.21.11.3692-3703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Devary Y, Rosette C, DiDonato JA, Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993;261(5127):1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- 41.Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446(7138):921–925. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- 42.Wan Y, Liu X, Kirschner MW. The anaphase-promoting complex mediates TGF-beta signaling by targeting SnoN for destruction. Mol Cell. 2001;8(5):1027–1039. doi: 10.1016/s1097-2765(01)00382-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.