Table 1.
Summary of parameters and outcomes of the PET/fMRI paradigm for putamen in two animals (M1 and M2)
| Study parameters and outcomes | Dose 1 | Dose 2 | Dose 3 | Dose 4 | ||||
| Study parameters* | ||||||||
| Injected RAC mass, μg/kg | 0.3 | 0.3 | 4.5 | 4.5 | 16 | 16 | 40 | 40 |
| Specific activity, μCi/nmol | 1,350 | 1,350 | 53 | 53 | 8.9 | 8.9 | 6 | 6 |
| Study outcomes | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 |
| BPND | 3.0 | 5.6 | 1.8 | 3.2 | 1.1 | 1.6 | 0.3 | 0.9 |
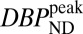
|
3.3 | 5.1 | 1.6 | 2.4 | 0.8 | 1.1 | 0.2 | 0.6 |
RAC occupancies†
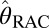 , % , % |
0 | 0 | 40 | 43 | 63 | 71 | 90 | 84 |
Peak RAC occupancies†
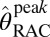 , % , % |
0 | 0 | 50 | 52 | 74 | 77 | 94 | 88 |
Peak 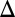 CBV, % CBV, % |
0.5‡ ± 0.3 | −1.1‡ ± 0.4 | 2.2 | 5.8 | 5.6 | 10 | 7.3 | 12 |
Average values from studies in two animals.
Occupancies are computed relative to dose 1.
Average CBV from repeated studies.
