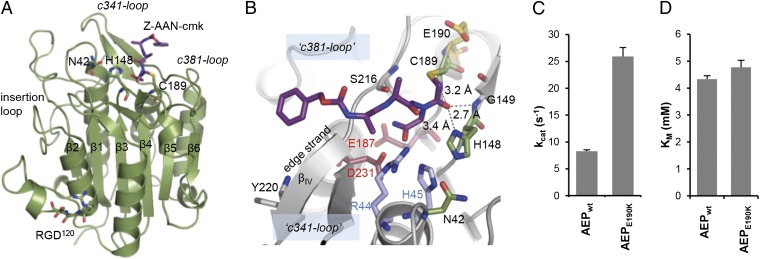
Fig. 1.
Substrate specificity and catalytic mechanism of legumain. (A) Legumain has a caspase-like overall structure. Active site residues (Cys189, His148, and Asn42) and the RGD120 motif are highlighted as green sticks, and the covalent Z-Ala-Ala-AzaAsn-chloromethylketone inhibitor in purple sticks. (B) Substrate specificity of legumain is determined by its zwitterionic S1 pocket. The Z-Ala-Ala-AzaAsn-cmk inhibitor (purple sticks) is covalently linked to the catalytic Cys189. The catalytic triad is represented by green sticks and residues forming the S1 pocket by red and blue sticks. Note the trivalent oxyanion pocket indicated by dashed lines. (C) Legumain activity critically depends on the local pKa of Cys189. The latter can be tuned by an E190K mutation, resulting in a fourfold increase in kcat at pH 5.5 as measured by the turnover of Bz-Asn-pNA. (D) KM values of active wild-type legumain (AEP) and E190K legumain toward the Bz-Asn-pNA substrate at pH 5.5 are virtually identical, indicating that the charge reversal affects exclusively the turnover number via pKa tuning of Cys189. Data in C and D are represented as mean ± SD of three experiments.