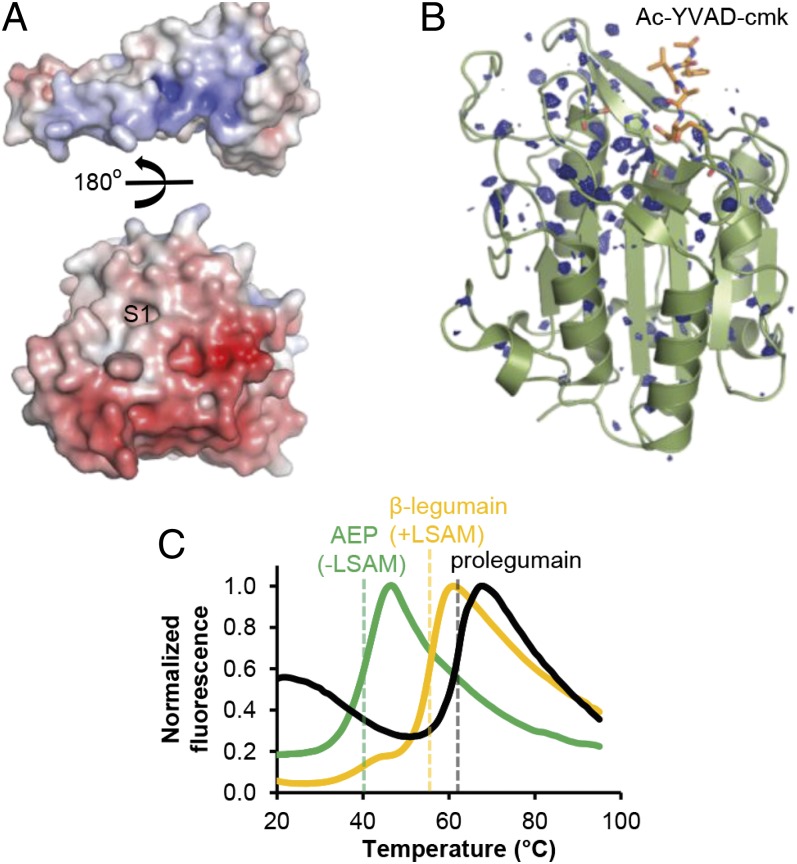
Fig. 3.
The LSAM domain stabilizes legumain via electrostatic interactions. (A) Color-coded electrostatic surface potential of the catalytic domain (red; negative charge) and the LSAM domain including helix APαV (blue; positive charge) calculated at pH 7.0. The catalytic domain is rotated relative to the view in Fig. 2A by 90° around the horizontal x axis, and the LSAM domain has been separated and rotated by 180° relative to the catalytic domain. (B) Isomorphous σA-weighted difference density FpH7.5–FpH5.0 of legumain (AEP) crystallized at pH 7.5 and pH 5.0. In either case, legumain was covalently inhibited with Ac-YVAD-cmk (orange sticks). Strong changes in X-ray diffraction, as evident by the difference density, reflect changes in protein dynamics and stability. These changes cluster at the area surrounding the active site that corresponds to the LSAM domain binding interface; this confined mobility suggests that LSAM stabilizes the protease domain at neutral pH. Contour level: 2.9 σ over the mean; catalytic residues are indicated as green sticks. (C) Thermal denaturation curves show a stabilization of AEP by the LSAM domain. Melting curves of AEP (lacking the LSAM domain), β-legumain (cleaved at Asn323, LSAM domain remains noncovalently bound to the catalytic domain), and prolegumain were measured at pH 6.5 by the Thermofluor method. Melting points are indicated by dashed lines.