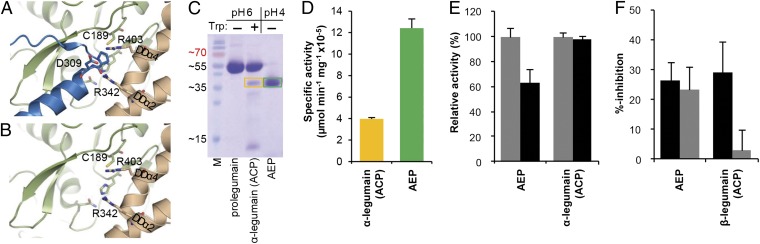
Fig. 4.
Prolegumain can develop carboxypeptidase activity upon cleavage of the AP. (A) The AP binds substrate-like to the nonprimed substrate binding cleft. The view to the active site is identical as in Fig. 1B. Catalytic residues are indicated as green sticks, and the double Arg-motif (Arg403 and Arg342) anchors the helix APαV via a salt bridge to Asp309. (B) View to the carboxypeptidase active site upon release of the AP. Arg403 and Arg342 are ideally positioned to anchor the C terminus of the substrate and, thereby, act as a molecular ruler for monopeptidyl peptidase activity, consistent with results from the peptide competition assay (Fig. S4 B and C). (C) SDS/PAGE after trypsin activation. After 2 h incubation of prolegumain with trypsin in a 1:50 molar ratio, approximately 12% of prolegumain were converted to an active enzyme (α-legumain, ∼36 kDa, orange box) as judged by using ImageJ (http://rsbweb.nih.gov/ij). In a control experiment, legumain was autoactivated via pH shift (AEP, green box). M, molecular mass marker in kilodaltons. (D) α-legumain carboxypeptidase activity can be generated via trypsin activation. Specific activity toward the fluorescent Z-AAN-AMC substrate is displayed. Orange, trypsin activated α-legumain; green, pH activated AEP. (E) Turnover of Z-AAN-AMC by pH 4.0 activated legumain (AEP) and trypsin activated α-legumain at pH 5.5 (gray bars) and pH 6.5 (black bars). Trypsin-activated legumain is not pH sensitive. Activity was normalized to maximum activity at pH 5.5. Data in D and E are represented as mean ± SD of three experiments. (F) Strict requirement for substrates with free carboxy terminus in ACP, but not AEP. Fluorogenic ACP activity can be competed off by peptides with a free P1' C terminus, H-AlaAlaAsn-Ala-OH; a single atom replacement (H-AlaAlaAsn-Ala-NH2; gray bars) completely abolishes the binding to ACP. By contrast, this exchange does not affect binding to the endopeptidase AEP.