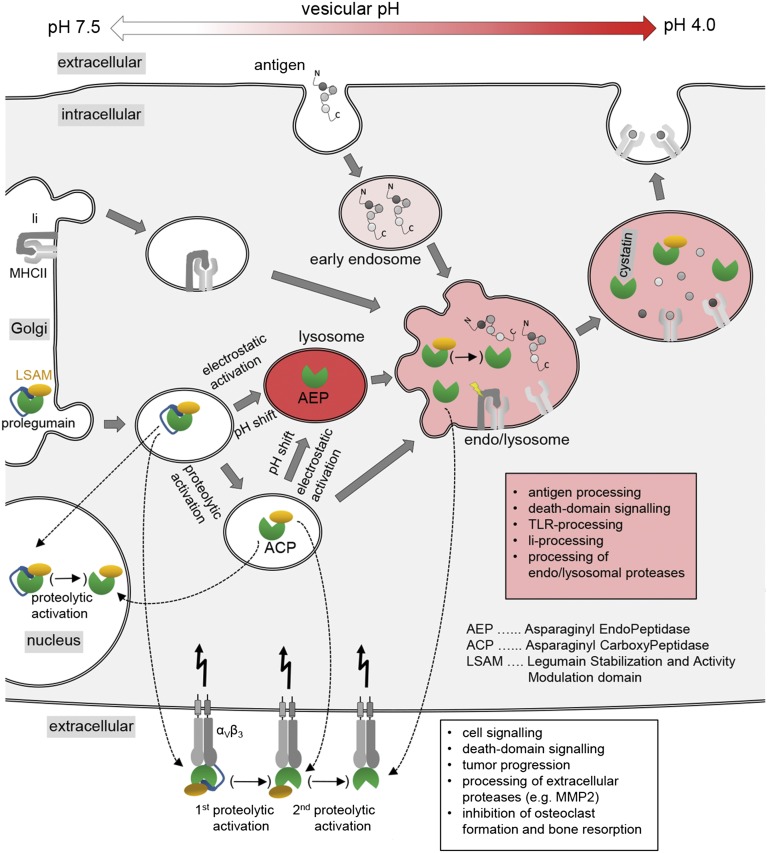
Fig. 6.
Multiple functions at multiple locations. Enzymatically active legumain was reported to exist in locations that are incompatible with AEP stability, i.e., pH ≥ 6. This contradiction can be reconciled by legumains three-modular architecture and its allosteric stabilization by the αVβ3 integrin receptor. After its posttranslational modification within the Golgi apparatus and vesiculation, prolegumain can be loaded onto αVβ3 receptors where a first proteolytic activation to ACP can take place, as necessary for its extracellular tasks (white text box). Integrin-stabilized AEP could be obtained by a second proteolytic activation event that would have to degrade the LSAM domain. Alternatively, transfer/fusion of prolegumain to lysosomal compartments with acidic pH (<5) will result in electrostatic release, and subsequent cleavage, of the prodomain resulting in AEP. By contrast, within near neutral compartments proteolytic activation generates ACP which will participate in endolysosomal roles (red text box).