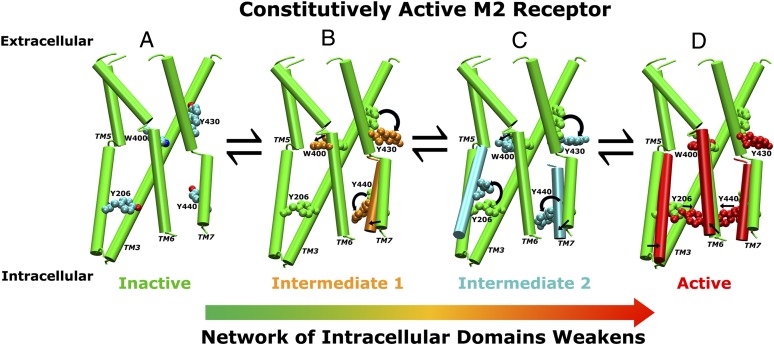
Fig. 5.
An allosteric activation pathway of the M2 receptor derived from aMD simulations. As a constitutively active GPCR, the apo M2 receptor exists in a conformational equilibrium of inactive, intermediate, and active states. (A) The inactive state with the TM3, TM5, TM6, and TM7 helices shown in cartoons and key residues Trp4006.48, Tyr4307.43, Tyr2065.58, and Tyr4407.53 in CPK representation. (B) Trp4006.48, Tyr4307.43, and Tyr4407.53 relocate their side chains during the receptor transition to the intermediate state. (C) Tyr2065.58 reorients the side chain from the initial position between TM3 and TM6 to the lipid-exposed side of TM6, resulting in an alternative intermediate conformation. (D) During final transition to the active sate, Tyr2065.58 and Tyr4407.53 relocate their side chains toward each other, forming a hydrogen bond, and the cytoplasmic end of TM6 tilts ∼6 Å outward away from the TM bundle. Activation of the receptor significantly reduces the network strength of the intracellular domains in the G-protein-coupling site, which apparently facilitates association of the G protein and further stabilization of the receptor active conformation.