Abstract
The biodiversity crisis is one of the greatest challenges facing humanity, but our understanding of the drivers remains limited. Thus, after decades of studies and regulation efforts, it remains unknown whether to what degree and at what concentrations modern agricultural pesticides cause regional-scale species losses. We analyzed the effects of pesticides on the regional taxa richness of stream invertebrates in Europe (Germany and France) and Australia (southern Victoria). Pesticides caused statistically significant effects on both the species and family richness in both regions, with losses in taxa up to 42% of the recorded taxonomic pools. Furthermore, the effects in Europe were detected at concentrations that current legislation considers environmentally protective. Thus, the current ecological risk assessment of pesticides falls short of protecting biodiversity, and new approaches linking ecology and ecotoxicology are needed.
Keywords: environmental impacts, environmental risk assessment, plant protection products, macroinvertebrates, spatial scale
The losses of biodiversity caused by anthropogenic activities during the past 50 y are unprecedented in human history (1). Despite general concern and several international initiatives (2–4), the current rate of biodiversity loss appears to be accelerating rather than slowing (5, 6). The future consequences of this crisis may be dramatic, as the latest analyses show that a planetary-scale ecosystem shift to an unknown and irreversible state may occur (7).
To date, no unequivocal link has been established between the measured exposure (i.e., the concentration of toxicants in the environment) and quantitative measures of regional biodiversity (i.e., the regional taxonomic richness pool). The only exceptions are two studies that addressed effects of salinity (8, 9). Hence, although chemical contaminants are well known as an important driver for biodiversity loss (1, 10–28), there is scarce empirical evidence to support such opinion for the large-scale taxonomic pools.
This problem holds true even for agricultural pesticides, which are among the best ecotoxicologically characterized and regulated groups of contaminants. Essentially, it remains unknown whether, to what degree, and at what concentrations pesticides cause the species losses at the regional scale. However, there are many investigations showing the effects on the local biodiversity-related parameters in both freshwater (16–23) and terrestrial systems (14, 15, 24–27). Thus, the previous studies with freshwater invertebrates reliably measured the aquatic pesticide concentrations and identified local (site-scale) changes in the abundance of the taxa specifically vulnerable to pesticides and structural community alterations, e.g., using the species-at-risk (SPEAR)pesticides indicator (16–21), or the abundance of separate species (22) (for different taxonomic groups, see ref. 23). Similarly, numerous investigations in the agroecosystems revealed various effects of pesticides on the terrestrial arthropod communities and their local biodiversity metrics (e.g., site- or farm-scale taxonomic richness; 14, 15, 24–27). Most of these impacts detected in both freshwaters and agroecosystems have a clear potential to propagate to alterations of the large-scale taxonomic pools, i.e., regional biodiversity (14, 15, 21), but such effects on the regional scale remained to be proven and quantified empirically.
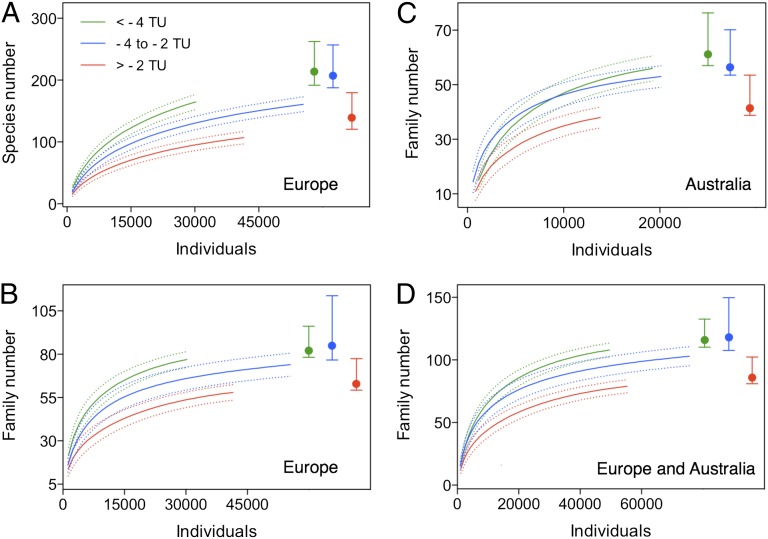
A fundamental measure of biodiversity is taxa richness, i.e., the number of taxa inhabiting a certain region or a set of sites. Despite its simplicity, taxa richness is an elusive quantity, as it is strictly dependent on the sampling effort and abundance: as more individuals and samples are collected, more species will be recorded (29, 30). Therefore, taxonomic richness can only be reliably measured using taxa accumulation or rarefaction curves. Such curves represent a relationship between the number of samples or individuals and the number of taxa recorded (29) (Fig. 1).
Fig. 1.
Taxonomic richness of stream macroinvertebrates in the site groups characterized by different levels of pesticide contamination. Data from Europe (A, species level, and B, family level), Australia (C, family level only), and the combined dataset (D). The richness is expressed as taxa rarefaction curves (left side of each graph), showing the dependence of the richness on the sampling efforts, and the richness estimator Chao 2 (right side of each graph), showing the richness predicted for an infinite number of samples. The site groups are reference (TU < –4), slightly contaminated (–4 < TU < –2), and highly contaminated (TU > –2).
Recently, the term “contaminant category richness” was introduced by Kefford et al. (9) to describe the taxa richness of stream invertebrates peculiar to different water salinity levels and quantified by rarefaction curves (8, 9). This richness is conceptually similar to richness in latitudinal, altitudinal, or marine barometric zones (31) and reflects the taxonomic pool of a large set of sites having a certain contamination level (9). The contaminant category richness is a measure of the regional richness constrained by the contamination level, and essentially, it represents the split of the regional taxonomic pool characterized by a certain contamination level. This approach differs fundamentally from the point richness or site-specific richness (i.e., the number of taxa per sample or site) that is commonly used, as the latter type of richness only reflects a small fraction of the taxonomic pool and, therefore, was suggested to be defined as taxon density (29).
In the present study, we applied the contaminant category richness to investigate the effects of pesticides on stream invertebrates using the data from Europe [Germany (16) and France (17)] and Australia [southern Victoria (18)]. These data were chosen as they included (i) exposure assessment using methods designed to capture episodic pesticide exposure, (ii) records of stream invertebrates, and (iii) data on the principal environmental factors that may confound the effects of pesticides. The taxonomic richness was compared across site groups characterized by different levels of pesticide contamination (i.e., contamination category). The contamination categories were as follows: 1, reference—sites with log-transformed toxic units (TUs) < –4; 2, slightly contaminated—sites with –4 < TU < –2; and 3, highly contaminated—sites with TU > –2 (following ref. 32). These three contamination categories correspond to <1/10,000, 1/10,000–1/100, and >1/100, respectively, of the median acute effect concentration (EC50) values for the reference species Daphnia magna (for details, see Materials and Methods).
Results and Discussion
The rarefaction analysis revealed significant differences in taxonomic richness among all three of the contamination categories for both the species- and family-level data from Europe, as indicated by the nonoverlapping 95% confidence intervals (Fig. 1 A and B). For Australia, the rarefaction analysis for family-level data only showed a difference between the highly contaminated category and both the reference and slightly contaminated categories (Fig. 1C). The curves based on the combined dataset were similar to those found for the European family-level data (Fig. 1D). The percentage decrease in taxonomic richness between the uncontaminated and highly contaminated categories ranged from 42% for the European species-level data to 27% for the Australian family-level data, as calculated for the highest numbers of samples available for all three of the site groups (Fig. 2).
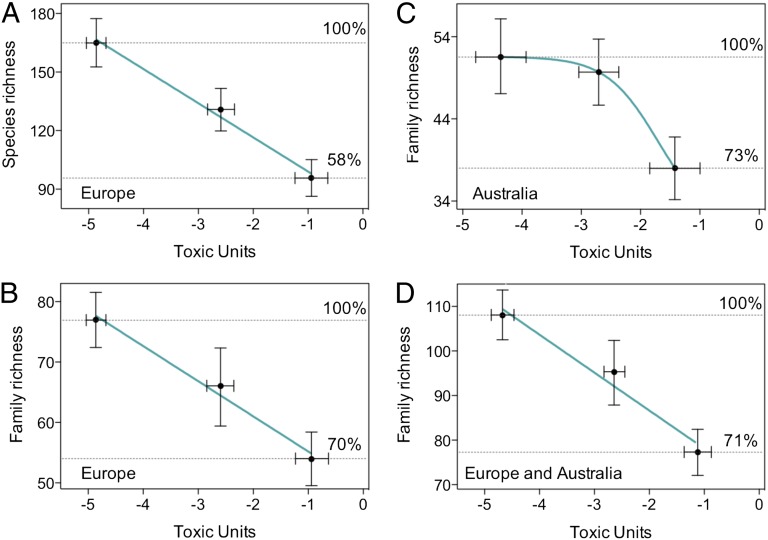
Fig. 2.
Concentration–response dependence between the mean pesticide concentration and mean overall taxa richness in the three site groups characterized by different levels of pesticide contamination. Data from Europe (A, species level, and B, family level), Australia (C, family level only), and the combined dataset (D). The taxa richness values are derived from the rarefaction curves (Fig. 1) for the highest number of samples available for all three groups for each case. The regression lines are derived by linear (A, B, and D) and log logistic (C) regression models to illustrate the trends. The dashed horizontal lines indicate the maximum and minimum mean richness and are marked with the percentages of maximum richness. The error bars indicate 95% confidence intervals.
The richness predicted for an infinite number of samples by the estimator Chao 2 differed significantly between the highly contaminated sites and both the reference and slightly contaminated sites for all datasets examined (Fig. 1). The differences between the empirical (i.e., the rarefaction curves, Fig. 1) and predicted richness (i.e., Chao 2 in Fig. 1, right upper corners) indicate that pesticides may cause severe declines in the abundance and/or localization of certain taxa rather than their full absence from contaminated sites. Such taxa can only be found through excessive sampling effort and are unlikely to sustain viable populations [i.e., sink populations (33)]. Therefore, the relatively weaker pesticide effects detected by the Chao 2 estimator should be interpreted with caution.
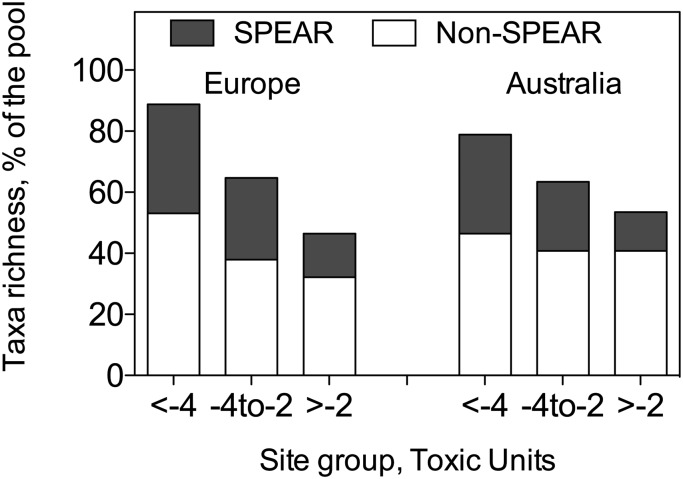
To discriminate the possible confounding factors from the effects of pesticides, we used two lines of analyses. First, we checked whether the observed declines in taxa richness are based on the losses of taxa that are particularly vulnerable to pesticides due to their high physiological sensitivity and combination of eco/biological traits following the classification of the highly pesticide-specific SPEAR approach (16–21, 32). We found that pesticide contamination was, indeed, associated with a decrease in the shares of pesticide-vulnerable taxa (Fig. 3). Thus, the observed losses in taxonomic diversity were, to a large degree, determined by the loss of those taxa specifically vulnerable to pesticides (for details, see Fig. S1 and Tables S1 and S2).
Fig. 3.
Taxa richness expressed as a percentage of the entire species pool and shares of the pesticide-vulnerable SPEAR taxa and not vulnerable Non-SPEAR taxa. The values are given for the site groups in Europe and Australia characterized by different level of pesticide contamination: reference (TU < –4), slightly contaminated (–4 < TU < –2), and highly contaminated (TU > –2).
Second, we analyzed whether any other available water quality and habitat variable would explain the differences between the three contamination categories (Tables S3 and S4), revealing only a significant difference in the electrical conductivity (a measure of salinity) of the water between the slightly and heavily contaminated sites in Australia. However, there was no consistent linear trend in water conductivity, with only the slightly contaminated sites having disproportionately low conductivity values (Fig. S2). Hence, water conductivity is very unlikely to be a major determinant of the observed diversity patterns.
Our results demonstrate that pesticides do produce pronounced negative effects on the regional biodiversity of stream invertebrates in both Europe and Australia. Furthermore, the effects on the taxa richness in Europe were identified in the contaminant category with TUs ranging from 1/10,000 to 1/100 of the model species D. magna EC50 (–4 < TU < –2; Figs. 1 and 2), i.e., at a concentration level that is considered to be protective by the current European regulation for agricultural pesticides (34, 35), which states that no effects should occur below 1/100 of the EC50 of Daphnia spp. or fish (for discussion, see ref. 21).
Thus, the current risk assessment standards and/or their implementation in agricultural practice are not protective for regional biodiversity of the stream invertebrates. These findings are in accordance with previous studies on the site-scale effects on the abundance of taxa specifically vulnerable to pesticides, as shown in a metaanalysis of eight studies (21). Importantly, the present analyses show that the effects previously identified on the site scale for pesticide-sensitive organisms are actually translated into the alteration of the entire taxonomic pools in the contamination categories.
The present outcomes indicate that the aim to reduce the rate of biodiversity loss and to meet the 2020 targets set by the Convention on Biological Diversity (3, 5, 36) is jeopardized for freshwater ecosystems. Our analysis shows that the pesticides most of which are currently in use in Europe and Australia may cause declines of up to 42% of the stream invertebrates’ species pools (Fig. 2). Such an extensive decline is comparable to the effects of other drivers and, as already demonstrated (17, 21), can be translated into functional impairments (1). Pesticide use has not decreased in the last decade (e.g., Eurostat Database; http://epp.eurostat.ec.europa.eu) and is predicted to increase in the next decades due to climate change (37) and thus may be a more important driver of biodiversity loss in the future.
The current prioritization of the biodiversity loss drivers may be misleading if pesticides are not considered. The measurement of the environmental concentrations of pesticides is difficult and expensive due to their episodic and low-level exposure and the multitude of substances (12, 38). Therefore, the actual effects of pesticides can easily be misattributed to other more “traditional” drivers (e.g., N and P levels and habitat degradation), which are better understood and can be more easily investigated.
If the aims of slowing the biodiversity loss rate (3, 4) and minimizing the effects of contaminants on biodiversity (34, 35) are to be achieved, the existing pesticide registration, methods of application to fields, and mitigation practices (e.g., nonsprayed buffer zones near waterways) should be developed toward more protective standards.
More generally, ecotoxicology as any applied ecological discipline should be matched to scales relevant for management practices. So with pesticides applied at the field scale and generally regulated at the national or supernational scale, ecotoxicology investigations should cover these scales. There is a clear need to better incorporate ecological theory and new large-scale-oriented approaches (e.g., 9, 16) to estimate and predict effects of contaminants across various spatial and temporal scales including the regional scale (12).
Materials and Methods
Datasets.
To investigate the effects of agricultural pesticides on the taxa richness of stream invertebrates, we used datasets for the effects of pesticides on macroinvertebrates in small streams in two different biogeographical regions of Europe, including a central plains region in Germany (16) and a western plains region in France (17), and in southern Victoria, Australia (18). The datasets include the results of extensive pesticide analyses based on methods that reflect short-term peak pesticide exposure (see below). The datasets also include information on macroinvertebrate communities (abundance of taxa), and basic water quality parameters (Tables S3 and S4). In all, the datasets comprise information on 48 and 24 sampling sites in Europe and Australia, respectively. The general characteristics of the streams investigated were as follows: current velocity ranging between 0.1 and 0.5 m/s, maximum stream depth of 0.8 m, no drying up in summer, no dredging in the present or past year, and presence of adjacent fields (except for several reference sites in Australia) with grape vines, orchards, berries, vegetables, corn, sugar beet, or oil-seed crops. The sites were evaluated with field surveys and maps to ensure that they had no wastewater treatment plants, industrial facilities, or mining drainage upstream. Thus, pollution other than from agricultural sources was unlikely (for details, see refs. 16–18).
Pesticide Sampling and Analyses.
The pesticide monitoring was designed to capture episodic runoff events, as this is a major input path for pesticides in small streams (16–21). The substances for the analyses were selected based on regulatory monitoring programs, pesticide use information from local agricultural advisory boards, and all other available information. Additionally, to select the most toxic compounds for the monitoring, the compounds were ranked according to their toxicity, as indicated by the 48-h acute median lethal concentration (LC50) for Daphnia magna taken from ref. 39 or the FOOTPRINT Pesticide Properties Database (http://sitem.herts.ac.uk/aeru/footprint/). The lists of measured pesticides differed between the study regions due to differences in the crops, pests, and pesticide authorization. The numbers of compounds analyzed were 21, 10, and 97 for Germany, France, and Australia, respectively (Table S5).
In Germany, two event-controlled runoff sampling systems were used: (i) an automated active sampler triggered by a conductivity decrease and water level increase and (ii) runoff-triggered 1-L bottle samplers passively triggered by a water level increase and retrieved after heavy rain events. The latter sampling system was also used in the study in France. In Australia, three methods were used: grab water sampling with a 1-L bottle, passive sampling using low-density polyethylene (LDPE) bags filled with 2,2,4-trimethylpentane (TRIMPS), and sediment samples. The TRIMPS passive samplers consisted of prefabricated LDPE membrane bags that were prerinsed overnight in 2,2,4-trimethylpentane and subsequently deployed for ∼28 d. The sediments were sampled using a dip net, wet-sieved on site to 64 μm, and decanted into a 1-L solvent-rinsed jar after a 15-min settling period (for details, see refs. 16–18). Although the sampling methods differed between the regions, the outcomes are comparable, as very similar relationships between the estimated pesticide toxicity in terms of the TUs and biotic endpoints were obtained (compared in ref. 21).
Expression of Pesticide-Related Water Toxicity.
To compare the toxicity associated with the pesticide concentrations measured in the sampling sites, the TUs were computed from the maximum peak water concentrations measured at each site (16):
where TU(D. magna) is the maximum toxic unit value of the n pesticides detected at the site considered, Ci is the concentration (in micrograms per liter) of pesticide i, and LC50i is the 48-h LC50 of pesticide i for D. magna (in micrograms per liter), as given in ref. 34 or Footprint database (for the extended discussion on applicability of this approach, see ref. 21).
Macroinvertebrate and Environmental Variables.
In Europe, macroinvertebrates were collected with a Surber sampler (area of 0.062 m2, four replicate samples collected randomly over a stream length of 50 m per site/date). The macroinvertebrates were sorted, counted, and identified to the lowest possible taxonomic level, which was the species/genus for most of the taxa (16, 17). In Australia, the macroinvertebrate sampling was conducted according to the rapid bioassessment method of the Environment Protection Authority Victoria (40) and involved taking a sample from the edge/pool habitat with a kick net and, where riffles were present, a kick net (41). The taxa were identified to the family level due to the lack of taxonomic information on lower levels for many taxonomic groups (18). The measured environmental variables are summarized in Tables S3 and S4. The measurements of the water physicochemical parameters and assessment of the habitat and landscape parameters were performed on site.
Species Richness Calculations and Data Analyses.
Taxonomic richness was quantified using the sample-based rarefaction curves calculated without replacement (Fig. 1) (in the terminology in ref. 24). The curves were calculated with 95% confidence intervals according to the analytical equations (42). Sample-based rarefaction was chosen to account for the natural levels of sample heterogeneity (patchiness) in the data. Following ref. 29, the sample-based rarefaction curves were plotted as a function of the cumulative number of individuals, not the cumulative number of samples, to avoid possible biases based on systematic differences in the mean number of individuals per sample. We used the classic richness estimator Chao 2 to predict the taxonomic richness for an infinite number of samples (43). This estimator generates asymmetrical confidence intervals that are based on the assumption that log(Sestimated − Sobserved) is normally distributed (where S is taxa richness). This assumption is reasonable in that the lower confidence bound cannot be less than the observed number of species (38, 39). The species richness calculations were performed using EstimateS 8.2 software (University of Connecticut, Storrs, CT; http://viceroy.eeb.uconn.edu/EstimateS/) (44).
To illustrate the concentration–response dependence between the estimated pesticide toxicity and regional taxa richness, we calculated a linear regression model between the mean TU per contamination-category site group and mean overall taxa richness in this site group (i.e., the three site groups characterized by different levels of pesticide contamination; Fig. 2). A nonlinear log logistic regression model was only fitted for the Australian data because it showed an obvious nonlinear relationship (Fig. 2C).
To determine whether the observed declines in the taxa richness are based on the losses of taxa that are particularly vulnerable to pesticides, we applied the SPEAR approach, which is known to have a high specificity with regard to pesticides (16–21, 27). The SPEAR approach divides the stream invertebrate taxa according to a binary classification including “species at risk” and “species not at risk” (where the “species” can be any taxonomic category, e.g., species, genus, family) according to the following biological traits: physiological sensitivity to organic toxicants, generation time, presence of aquatic stages in water during the maximum pesticide use period, and migration abilities. We calculated the fractions of the SPEAR taxa in the taxonomic pools of the three site groups characterized by different levels of pesticide contamination (i.e., contamination categories) to ascertain whether the declines in the taxa richness are based on the losses of the SPEAR taxa (Fig. 3).
To determine whether factors other than pesticides can explain the observed taxa richness patterns, we compared the three groups of the sites with different pesticide contamination levels with respect to the available physicochemical water characteristics and habitat and landscape parameters (Tables S3 and S4). The comparisons were performed with a nonparametric multiple comparison test of the Behrens–Fischer type, followed by a Holm–Bonferroni correction. A nonparametric test was selected due to violations of normality identified by the Kolmogorov–Smirnov test. In addition, a Levene test was performed to check for differences in the variance between the categories. A high variation in habitat/water quality variables may lead to a greater number of species as a result of a wider niche. However, this latter test revealed no statistically significant differences between the categories.
The statistical computations were performed using the open-source software package R, version 2.7 for Mac OS X (www.r-project.org) and Prism 5.0b for Mac OS X (GraphPad Software).
Supplementary Material
Acknowledgments
We are grateful to everyone who helped with the fieldwork and the associated chemical analyses. We thank Graham Pyke for valuable comments on the manuscript. This work was supported by the Helmholtz Association of German Research Centers (Project ECOLINK, HRJRG-025). A visit to Germany by B.J.K. was funded by the University of Technology Sydney's International Researcher Development Scheme. Melbourne Water funded the collection of the pesticide data in the Australian study.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305618110/-/DCSupplemental.
References
- 1.Millenium Ecosystem Assessment . Ecosystems and Human Well-Being: Biodiversity Synthesis. Washington, DC: World Resources Institute; 2005. [Google Scholar]
- 2. United Nations (2008) Millennium Development Goals Indicators. Available at http://unstats.un.org/unsd/mdg/Host.aspx? Content=Indicators/OfficialList.htm. Accessed June 1, 2012.
- 3.Secretariat of the Convention on Biological Diversity . Global Biodiversity Outlook 3. Montreal: CBD; 2010. [Google Scholar]
- 4. Secretariat of the Convention on Biological Diversity (2003) Handbook of the Convention on Biological Diversity (Earthscan, CBD, London)
- 5.Butchart SHM, et al. Global biodiversity: Indicators of recent declines. Science. 2010;328(5982):1164–1168. doi: 10.1126/science.1187512. [DOI] [PubMed] [Google Scholar]
- 6.Walpole M, et al. Ecology. Tracking progress toward the 2010 biodiversity target and beyond. Science. 2009;325(5947):1503–1504. doi: 10.1126/science.1175466. [DOI] [PubMed] [Google Scholar]
- 7.Barnosky AD, et al. Approaching a state shift in Earth’s biosphere. Nature. 2012;486(7401):52–58. doi: 10.1038/nature11018. [DOI] [PubMed] [Google Scholar]
- 8.Kefford BJ, Nugegoda D, Metzeling L, Fields EJ. Validating species sensitivity distributions using salinity tolerance of riverine macroinvertebrates in the southern Murray-Darling Basin (Victoria, Australia) Can J Fish Aquat Sci. 2006;63(8):1865–1877. [Google Scholar]
- 9.Kefford BJ, et al. The definition of species richness used by species sensitivity distributions approximates observed effects of salinity on stream macroinvertebrates. Environ Pollut. 2011;159(1):302–310. doi: 10.1016/j.envpol.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Rockström J, et al. A safe operating space for humanity. Nature. 2009;461(7263):472–475. doi: 10.1038/461472a. [DOI] [PubMed] [Google Scholar]
- 11.Vörösmarty CJ, et al. Global threats to human water security and river biodiversity. Nature. 2010;467(7315):555–561. doi: 10.1038/nature09440. [DOI] [PubMed] [Google Scholar]
- 12.Beketov MA, Liess M. Ecotoxicology and macroecology—time for integration. Environ Pollut. 2012;162:247–254. doi: 10.1016/j.envpol.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 13.McMahon TA, et al. Fungicide-induced declines of freshwater biodiversity modify ecosystem functions and services. Ecol Lett. 2012;15(7):714–722. doi: 10.1111/j.1461-0248.2012.01790.x. [DOI] [PubMed] [Google Scholar]
- 14.Geiger F, et al. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl Ecol. 2010;11(2):97–105. [Google Scholar]
- 15.Gibbs KE, Mackey RL, Currie DJ. Human land use, agriculture, pesticides and losses of imperiled species. Divers Distrib. 2009;15(2):242–253. [Google Scholar]
- 16.Liess M, Von Der Ohe PC. Analyzing effects of pesticides on invertebrate communities in streams. Environ Toxicol Chem. 2005;24(4):954–965. doi: 10.1897/03-652.1. [DOI] [PubMed] [Google Scholar]
- 17.Schäfer RB, et al. Effects of pesticides on community structure and ecosystem functions in agricultural streams of three biogeographical regions in Europe. Sci Total Environ. 2007;382(2–3):272–285. doi: 10.1016/j.scitotenv.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 18.Schäfer RB, et al. Effects of pesticides monitored with three sampling methods in 24 sites on macroinvertebrates and microorganisms. Environ Sci Technol. 2011;45(4):1665–1672. doi: 10.1021/es103227q. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen JJ, Baattrup-Pedersen A, Larsen SE, Kronvang B. Local physical habitat quality cloud the effect of predicted pesticide runoff from agricultural land in Danish streams. J Environ Monit. 2011;13(4):943–950. doi: 10.1039/c0em00745e. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen JJ, Wiberg-Larsen P, Baattrup-Pedersen A, Friberg N, Kronvang B. Stream habitat structure influences macroinvertebrate response to pesticides. Environ Pollut. 2012;164:142–149. doi: 10.1016/j.envpol.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Schäfer RB, et al. Thresholds for the effects of pesticides on invertebrate communities and leaf breakdown in stream ecosystems. Environ Sci Technol. 2012;46(9):5134–5142. doi: 10.1021/es2039882. [DOI] [PubMed] [Google Scholar]
- 22.Friberg N, Lindstrom M, Kronvang B, Larsen SE. Macroinvertebrate/sediment relationships along a pesticide gradient in Danish streams. Hydrobiologia. 2003;494(1–3):103–110. [Google Scholar]
- 23.Schäfer RB, van den Brink PJ, Liess M. In: Impacts of Pesticides on Freshwater Ecosystems. Ecological Impacts of Toxic Chemicals. Sánchez-Bayo F, van den Brink PJ, Mann RM, editors. Bussum, The Netherlands: Bentham Science Publishers Ltd.; 2011. [Google Scholar]
- 24.Cattaneo MG, et al. Farm-scale evaluation of the impacts of transgenic cotton on biodiversity, pesticide use, and yield. Proc Natl Acad Sci USA. 2006;103(20):7571–7576. doi: 10.1073/pnas.0508312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tilman D, et al. Forecasting agriculturally driven global environmental change. Science. 2001;292(5515):281–284. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- 26.Teodorescu I, Cogalniceanu D. Rapid assessment of species diversity changes after pesticide application in agricultural landscapes. Appl Ecol Environ Res. 2006;4(1):55–62. [Google Scholar]
- 27.Colignon P, Hastir P, Gaspar C, Francis F. Effects of insecticide treatments on insect density and diversity in vegetable open fields. Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet. 2001;66(2a):403–411. [PubMed] [Google Scholar]
- 28.Leslie TW, Biddinger DJ, Rohr JR, Fleischer SJ. Conventional and seed-based insect management strategies similarly influence nontarget coleopteran communities in maize. Environ Entomol. 2010;39(6):2045–2055. doi: 10.1603/EN10049. [DOI] [PubMed] [Google Scholar]
- 29.Gotelli NJ, Colwell RK. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett. 2001;4(4):379–391. [Google Scholar]
- 30.Chao A, Colwell RK, Lin CW, Gotelli NJ. Sufficient sampling for asymptotic minimum species richness estimators. Ecology. 2009;90(4):1125–1133. doi: 10.1890/07-2147.1. [DOI] [PubMed] [Google Scholar]
- 31.Colwell RK, Lees DC. The mid-domain effect: Geometric constraints on the geography of species richness. Trends Ecol Evol. 2000;15(2):70–76. doi: 10.1016/s0169-5347(99)01767-x. [DOI] [PubMed] [Google Scholar]
- 32.Beketov MA, et al. SPEAR indicates pesticide effects in streams—comparative use of species- and family-level biomonitoring data. Environ Pollut. 2009;157(6):1841–1848. doi: 10.1016/j.envpol.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Pulliam HR. Sources, sinks, and population regulation. Am Nat. 1988;132(5):652–661. [Google Scholar]
- 34. European Council (1991) European Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market (European Union, Brussels)
- 35. European Commission (2009) European Commission Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides (European Union, Brussels)
- 36.Perrings C, et al. Conservation. Ecosystem services for 2020. Science. 2010;330(6002):323–324. doi: 10.1126/science.1196431. [DOI] [PubMed] [Google Scholar]
- 37.Kattwinkel M, Kühne JV, Foit K, Liess M. Climate change, agricultural insecticide exposure, and risk for freshwater communities. Ecol Appl. 2011;21(6):2068–2081. doi: 10.1890/10-1993.1. [DOI] [PubMed] [Google Scholar]
- 38.Schwarzenbach RP, et al. The challenge of micropollutants in aquatic systems. Science. 2006;313(5790):1072–1077. doi: 10.1126/science.1127291. [DOI] [PubMed] [Google Scholar]
- 39. Tomlin CDS (2001) The e-Pesticide Manual on CD, Version 2.1 (British Crop Protection Council, Hampshire, UK), 12th Ed.
- 40.EPA-Australia . Rapid Bioassessment Methodology for Rivers and Streams. Melbourne: Environment Protection Authority; 2003. [Google Scholar]
- 41.Chessman BC. Rapid assessment of rivers using macroinvertebrates: A procedure based on habitat-specific sampling, family level identification and a biotic index. Aust J Ecol. 1995;20(1):122–129. [Google Scholar]
- 42.Colwell RK, Mao CX, Chang J. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology. 2004;85(10):2717–2727. [Google Scholar]
- 43.Chao A. Nonparametric estimation of the number of classes in a population. Scand J Stat. 1984;11(4):265–270. [Google Scholar]
- 44.Colwell RK. EstimateS 8.2 User's Guide. Storrs, CT: Univ of Connecticut; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.