Abstract
Background
Loss or disruption of Kit+-interstitial cells of Cajal (ICC) capable of generating pacemaker activity has been implicated in the development of numerous gastrointestinal motility disorders. We sought to develop a model where ICC could be allotransplanted into intestines naturally devoid of these cells.
Methods
Enzymatically dispersed cells from the intestinal tunica muscularis of Kit+/copGFP and KitV558Δ/+ gain-of-function mice were allotransplanted into myenteric plexus regions of W/WV mutant intestines that lack ICC at the level of the myenteric plexus (ICC-MY) and pacemaker activity. Immunohistochemical analysis fate mapped the development of ICC-MY networks and intracellular microelectrode recordings provided evidence for the development of functional pacemaker activity.
Key Results
Kit+-ICC developed into distinct networks at the level of the myenteric plexus in organotypic cultures over 28 days and displayed robust rhythmic pacemaker activity.
Conclusions and Inferences
This study demonstrates the feasibility of allotransplantation of ICC into the myenteric region of the small intestine and the establishment of functional pacemaker activity into tissues normally devoid of ICC-MY and slow waves, thus providing a possible basis for the therapeutic treatment of patients where ICC networks have been disrupted due to a variety of pathophysiological conditions.
Keywords: Interstitial cells of Cajal, pacemaker, electrical slow waves, allotransplantation
INTRODUCTION
Interstitial cells of Cajal (ICC) are now established as critical elements in the regulation of gastrointestinal (GI) motility, having been implicated in playing crucial roles as pacemakers1–3 and as mediators of enteric motor neurotransmission.4–7 Most regions of the GI tract display spontaneous electrical depolarization/repolarization events known as slow waves. In the small intestine slow waves are initiated within ICC networks at the level of the myenteric plexus (ICC-MY)3, 8 and spread passively to the adjacent electrically coupled smooth muscle cells.8–10 Depolarization of neighboring smooth muscle cells elicits excitation-contraction coupling and activation of the contractile apparatus. Slow waves therefore act to organize and pace the phasic contractile activity allowing for segmentation and propagation within the small intestine.11
Disruption or loss of ICC has been implicated in a host of GI motility disorders including achalasia,12, 13 slow transit constipation,14, 15 intestinal pseudoobstruction,16, 17 Crohn’s disease,18 inflammation19, 20 and diabetic gastroparesis21–24 together with natural processes such as aging.25, 26 The ability to manipulate and restore ICC networks in patients with pathophysiological conditions that have led to their loss would likely contribute to the re-establishment of normal GI motility. However, to date such experiments have not been performed in patients or in animal models and it is not known whether ICC networks can be restored in regions of the GI tract previously devoid of these cells.
Development and maintenance of ICC networks is dependent on c-Kit/stem cell factor signaling.27–29 The W locus is allelic for c-Kit and there are a number of mutations of the W locus exist, in which the tyrosine kinase activity of c-Kit is lost or compromised.30 Mutations within the W locus, such as in W/WV mutant mice, displaying reduced tyrosine kinase activity and have a well-characterized loss or absence of ICC-MY in the small intestines with a resultant loss of pacemaker activity.1, 2 These mutants provide an excellent model system in which to test the validity of restoring ICC and pacemaker function in a region od the GI tract that lacks these cells and function.
We hypothesized that allotransplantation of ICC into intestines where they are absent (i.e. W/WV mutants) may allow for their functional establishment to occur. The present study revealed that ICC can populate tissues and establish pacemaker activity where they were originally absent, thus providing a possible basis for the therapeutic treatment of patients where ICC networks have been disrupted due to a variety of pathophysiological conditions.
METHODS
Animals
W/WV mice (30–60 days old) were obtained from The Jackson Laboratory (Bar Harbor, MN, USA). Mutant KitV558Δ/+ mice were provided by Peter Besmer (Sloan Kettering, NY) and Kit+/copGFP and wildtype mice were produced at the University of Nevada.24 Animals used for these studies were maintained and the experiments performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and the IACUC at the University of Nevada approved all procedures used.
Tissue preparation and organotypic culture
W/WV mice were euthanized following sedation with isoflurane and cervical dislocation. The entire small intestine was removed and placed in oxygenated cold (4°C) Krebs–Ringer’s buffer (KRB) for further dissection.
The intestines were opened along the mesenteric border and luminal contents washed away with KRB. After removal of the mucosa, strips of longitudinal muscle along the anti-mesenteric border, were dissected from the underlying circular muscle of both jejunum and ileum to expose the myenteric plexus region. Sections of tissue (5mm2) were pinned to Sylgard elastomer-coated bases of sterile 35 mm polypropylene dishes (Corning Glass Works, Corning, NY, USA), with the serosal side of the longitudinal muscle facing upwards. The muscles were preincubated in smooth muscle growth media (SMGM; Clonetics, San Diego, CA, USA) at 37°C for 1h, prior to the addition of dispersed intestinal cells (50,000 cells/20μl SMGM/tissue section). Tissues were subsequently incubated at 37°C in a humidified atmosphere (90%) of 95% O2–5% CO2, supplemented with 2% antibiotic-antimycotic (Gibco, Grand Island, NY, USA) and stem cell factor (5ng/ml, Sigma) for periods up to 28 days with culture media changed every second day. Control tissues were cultured in the absence of seeded cells. Organotypic cultures were examined at 4 specific time points (10,14,21&28 days).
Cell Preparation
Jejunum and ileum muscle strips from either Kit+/copGFP or KitV558Δ/+ intestines from P10 animals were equilibrated in Ca2+-free Hanks’ solution for 20 min and cells were dispersed,31 and passed through a Celltrics® 100 μm (Partec) filter to obtain a single cell suspension. Cells were centrifuged at 1000 rpm (5 min, 4°C) and diluted to the appropriate volume (50,000 cells in 20μl) in SMGM prior to seeding onto recipient organotypic cultures. After 30 minutes (enough time to let the cells settle onto the donor tissue) the media volume was made up to 2ml per dish. Dishes were gently handled throughout all procedures. Three experimental procedures were utilized for allotransplantation studies (i) W/WV intestines seeded with Kit+/copGFP derived cells. (ii) W/WV intestines seeded with KitV558Δ/+ derived cells and (iii) W/WV intestines cultured with just SMGM (control).
Electrophysiological experiments
Intracellular microelectrode recordings were performed in the presence of nifedipine to maintain cellular impalements as previously described.2 It has previously been shown that nifedipine does not affect slow waves in the small intestine of the mouse.2
Solutions and drugs
The electrophysiological bath chamber was constantly perfused with oxygenated Krebs–Ringer’s buffer (KRB) of the following composition (mM):NaCl 118.5;KCl 4.5;MgCl2 1.2;NaHCO3 23.8;KH2PO4 1.2;dextrose 11.0;CaCl2 2.4. The pH of the KRB was 7.3–7.4 when bubbled with 97% O2–3% CO2 at 37±0.5°C. Muscles were left to equilibrate for at least 3h prior to impalements. Nifedipine (Sigma; St Louis, MO, USA) was dissolved in ethanol and added to KRB at a concentration of 1μM.
Immunohistochemistry
To examine allotransplantation of Kit+-ICC into cultured W/WV intestines, dispersions from the tunica muscularis of Kit+/copGFP mice were used as a reporter to follow the fate of ICC. A second immunohistochemical approach with Image iT™ signal enhancer was also used to identify Kit+-ICC, as it reduced non-specific immunofluorescence. Organotypic cultures and P10 intestines were fixed in paraformaldehyde (4% w/v in 0.1M PBS for 15 min at 24°C). After fixation, tissues were washed (24h, x2) in PBS (0.01M, pH 7.2 at 4°C). Tissues were permeabilized in 0.5% Triton-X 100 (Sigma) for 2 minutes and rinsed (X3) in PBS (0.01M, pH 7.2). Image iT™ signal enhancer (200μl; Invitrogen) was applied to tissues for 30 min at 24°C, washed thoroughly in PBS (0.01M, pH 7.2) and blocked in bovine serum albumin (1% in PBS at room temperature for 1h). To identify ICC, tissues were incubated for 48h at 4°C in a goat polyclonal anti-mouse stem cell factor receptor (2μg/ml; R&D Systems Inc., Minneapolis, MN, USA) in 0.01M PBS containing Triton-X 100 (0.5%). Immunoreactivity was detected using Alexa Fluor-488 or Alexa Fluor-594 donkey anti-goat secondary antibody (1:1000 in PBS, 1h at room temperature; Invitrogen). Cryostat sections were prepared in a similar manner as whole mounts. Before mounting, tissues were washed overnight in 0.01M PBS. Controls were performed in the absence of primary or secondary antibodies. Tissues were examined using a LSM510 Meta (Zeiss) or Fluoview FV1000 confocal microscope (Olympus). Confocal micrographs of wholemounts were digital composites of the Z-series of scans (0.5μm optical sections, 10–50μM thick). Final images were constructed using FV10-ASW 2.1 software (Olympus) and Image-J software (NIH).
Statistical analysis
Data are expressed as means ± standard errors of the mean. Differences in the data were evaluated by unpaired Student’s t test. P values <0.05 were taken as statistically significant. The “n values” reported in the text refer to the number of muscles used for each protocol. Each muscle was taken from a separate animal. At least 3 recordings were performed on each tissue.
RESULTS
Development of ICC following transplantation
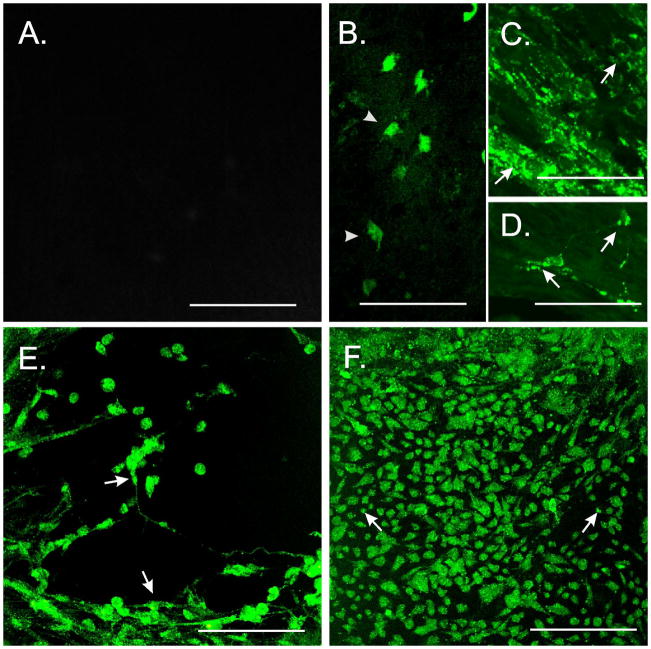
Examination of W/WV non-transplanted intestinal tissues revealed a population of ICC at the deep muscular plexus (ICC-DMP) as previously described,7 however Kit+-ICC at the level of the myenteric plexus (ICC-MY) were not observed (Fig. 1A). Associated with the absence of ICC-MY was a lack of slow waves (Fig. 1B). Examination of W/WV tissues that were organotypically cultured with intestinal tunica muscularis cells (50,000 cells/20 μl) revealed the time-dependent development of Kit+-ICC networks at the myenteric plexus (Fig. 2).
Figure 1.
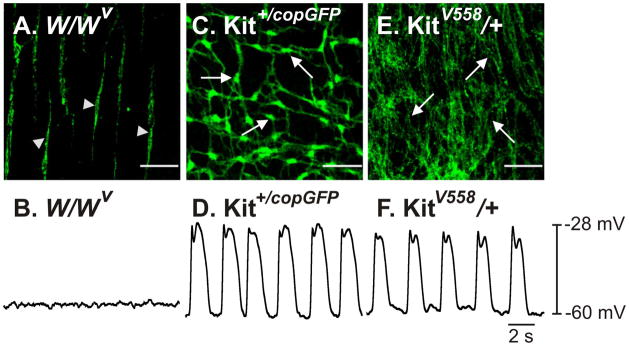
Presence or absence of ICC-networks and pacemaker activity in the intestines of W/WV, Kit+/copGFP and KitV558Δ/+ mutant mice. (A) Absence of ICC-MY, but not ICC-DMP (arrowheads) in W/WV intestines is associated with a lack of slow waves (B). The intestines of Kit+/copGFP mice display normal ICC-MY networks (C, arrows) and slow wave activity (D). ICC-MY are hyperplastic in KitV558Δ/+ mutants (E, arrows) but generate normal slow wave activity (F). Scale bars in panels A, C&E represent 50μm.
Figure 2.
Fate-mapping ICC-MY development in W/WV intestines following allotransplantation. (A) ICC-MY were not observed in control non-allotransplanted W/WV intestines. (B) Isolated copGFP+-ICC within the myenteric region of W/WV SI displaying short projections (arrowheads) 7d after allotransplantation of Kit+/copGFP cells. (C&D) After 14d, islands of copGFP+-ICC were observed and cells possessed a greater number of projections that contacted adjacent ICC (arrows). (E) Development of clusters of Kit+ cells characteristic of mature ICC 28d after allotransplantation of Kit+/copGFP cells. These cells displayed multipolar projections and contacted projections from neighboring ICC (arrows). (F) Allotransplantation of dispersed tunica muscularis cells derived from KitV558Δ/+ cells led to a more widespread distribution of Kit+-ICC at the myenteric plexus. Despite the increased density these Kit+-ICC presented as multipolar cells projecting shortened processes, which only occasionally formed contacts with each other (arrows). Scale bars represent 50 μm.
Using enzymatic dispersions from Kit+/copGFP mice as a means to follow the fate of allotransplanted ICC (Figs. 1C&D) revealed the presence of isolated individual Kit+-cells within 7d (Fig. 2B). These cells possessed a rounded appearance and had few projections. After 14d, islands of Kit+-ICC were observed and cells possessed a greater number of projections that contacted adjacent ICC (Figs. 2C&D). By 28d clusters of Kit+ cells displayed multipolar projections characteristic of mature ICC, which made contact with neighboring ICC (Fig. 2E).
Allotransplantation of dispersed tunica muscularis cells derived from KitV558Δ/+ intestines (Fig. 1E), to evaluate the importance of stem cell factor signaling for the establishment of ICC in donor tissues, lead to a more widespread distribution of Kit+-ICC-MY (Fig. 2F). Despite the increased density these Kit+-ICC presented as multipolar cells exhibiting numerous short processes, which only occasionally formed contacts with each other (Fig. 2F).
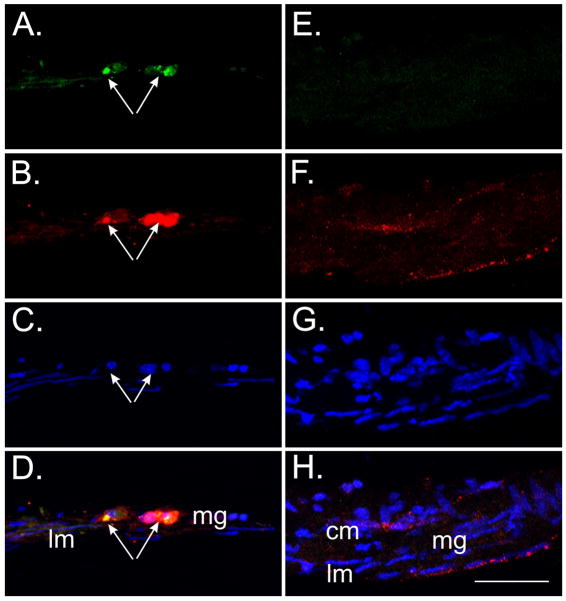
To determine whether dispersed tunica muscularis cells would allotransplant into different regions of the intestinal wall, experiments were performed on cells that were seeded onto (i) the serosal surface of the longitudinal layer, (ii) within the myenteric region and (iii) on the submucosal surface of circular muscle layer. Interestingly, it was only the cells that were seeded onto the myenteric region that developed into distinguishable networks. Cells that were seeded on the serosal or submucosal surfaces did not develop into Kit+-networks. Cryostat sections confirmed that cells preferentially grew when exposed to the myenteric region of intestines (Fig. 3A–D) and not when the cells were seeded on the serosal or submucosal surfaces (Fig. 3E–H).
Figure 3.
Kit+/copGFP cells preferentially grew within the myenteric plexus region but not on the submucosa or serosal surfaces. (A) Cryostat cross-section of W/WV SI revealing Kit+/copGFP cells (green, arrows) within the myenteric region. (B) The Kit+/copGFP cells were also Kit+ (red, arrows). (C) 4′,6-diamidino-2-phenylindole (DAPI) labeling of nuclei (blue) within Kit+/copGFP cells. Nuclei of the underlying longitudinal muscle are also visible in this section. (D) Merged image of panels A–C. mg refers to myenteric region, lm to longitudinal muscle layer. (E–H) Kit+/copGFP cells did not grow on intestinal tissues when the myenteric plexus region was not exposed. (E) Absence of Kit+/copGFP and (F) Kit+ cells on the submucosal or serosal surfaces. (G–H) DAPI labeling showing circular (cm) and longitudinal muscle (lm) layers. Scale bar in panel H = 50 μm and applies to all panels.
Cellular phenotypes in enzymatic dispersions of intestinal tissues
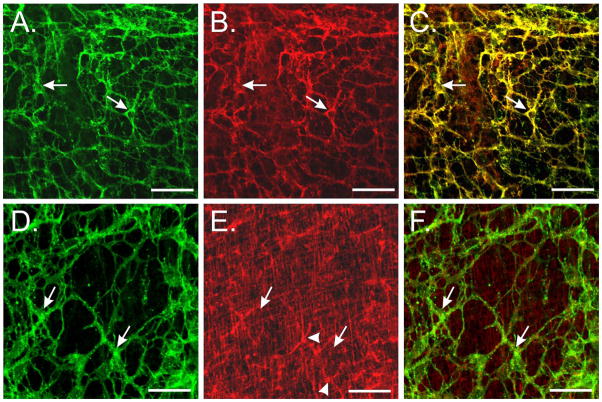
It has previously been shown that the tunica muscularis of the murine stomach contains a rare number of KitlowCD34+CD44+ cells that have been suggested may contribute to the regeneration and maintenance of mature ICC networks. These rare clusters of small rounded cells were reported to exist in the myenteric region, on the submucosal surface of the circular layer and on the serosal surface of the longitudinal muscle.32 It is possible that enzymatic dispersions of intestinal tunica muscularis contained these KitlowCD34+CD44+ cells that have been proposed to be progenitor ICC. We therefore examined the small intestines of wildtype animals from several different age groups (P0–P30) for expression of CD34+ and CD44+ cells and performed double labeling with Kit immunohistochemistry. CD44 immunohistochemistry revealed networks of cells resembling ICC-MY (Fig. 4B). Double labeling with Kit revealed cellular co-localization with CD44 (Fig. 4C) providing evidence that Kit+-ICC express CD44. Kit labeled the same population of cells that were copGFP+ within the tunica muscularis, except for rare rounded mast cells (not shown).
Figure 4.
Characterization of Kit+, CD44+ and CD34+ cells in the murine small intestine. (A&D) Identification of Kit+-ICC networks (arrows) in intestines of wildtype mice (green, arrows). Kit labeled an identical population of CopGFP+ cells. (B) CD44 immunohistochemical analysis revealed a network of cells resembling ICC-MY (arrows). (C) Double labeling with Kit revealed cellular co-localization with CD44 in ICC-MY (yellow, arrows). (E) CD34 immunohistochemistry labeled smooth muscle cells within the circular and longitudinal layers (arrows) and a stellate or multi-polar cell population (arrowheads), likely to represent fibroblast-like cells. Examination of large areas of tissues did not reveal the presence of groups of small rounded KitlowCD34+ cells. (F) Double labeling (merged images) of Kit and CD34 did not reveal cellular co-localization (arrows) in the multi-polar cells. Scale bars represent 50μm in all panels.
CD34 immunohistochemistry revealed labeling of spindle shaped smooth muscle cells within the circular and longitudinal layers. A second population of stellate or multipolar cells were also CD34+ and likely represent fibroblast-like cells in the small intestine (Fig. 4E).33 Examination of large areas of the tunica muscularis (≤15cm2 in P30 animals) did not reveal the presence of clusters of rounded cells resembling those described in the stomach.32 Double labeling of CD34 and Kit did not display any cellular co-localization suggesting that Kit+-ICC were not CD34+ (Fig. 4F). Examination of large areas of tissues along the submucosal surface of the circular layer and the serosal surface also failed to identify clusters of small rounded cells.
Mitotic division of Kit+-ICC as a source of self-perpetuation
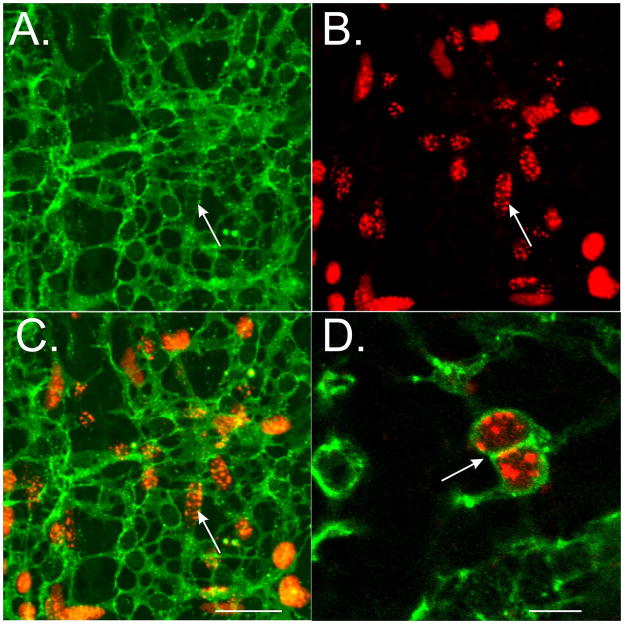
The failure to identify clusters of CD34+CD44+ cells within the tunica muscularis of the intestine and the uniform growth of Kit+ cells over the time-frame examined suggested that mature Kit+-ICC have the capability to undergo mitosis. We performed a series of experiments to examine this possibility in ICC-MY. Organotypically cultured intestinal muscles from animals of different ages P1–P10 were exposed to BrdU (24h), fixed and double labeled with antibodies against Kit. Examination of tissues revealed that Kit+-ICC-MY incorporated BrdU (Fig. 5), suggesting that differentiated Kit+-ICC possess the ability to mitotically divide.
Figure 5.
Mitotic division of differentiated ICC. BrdU incorporation demonstrates that differentiated Kit+-ICC undergo mitotic division. (A) Kit+-ICC networks in a P10 wildtype mouse (arrow). (B) BrdU incorporation of cells at the myenteric plexus (arrow). (C). Double labeling of Kit revealed cellular co-localization with BrdU labeling (arrow). (D) Higher magnification demonstrating the incorporation of BrdU into differentiated Kit+-ICC. Note the presence of anastomosing projections, a morphological feature of mature ICC (arrow). Scale bar = 50 μm in C for panels A–C. Scale bar in D = 25 μm.
Restoration of Slow Wave activity
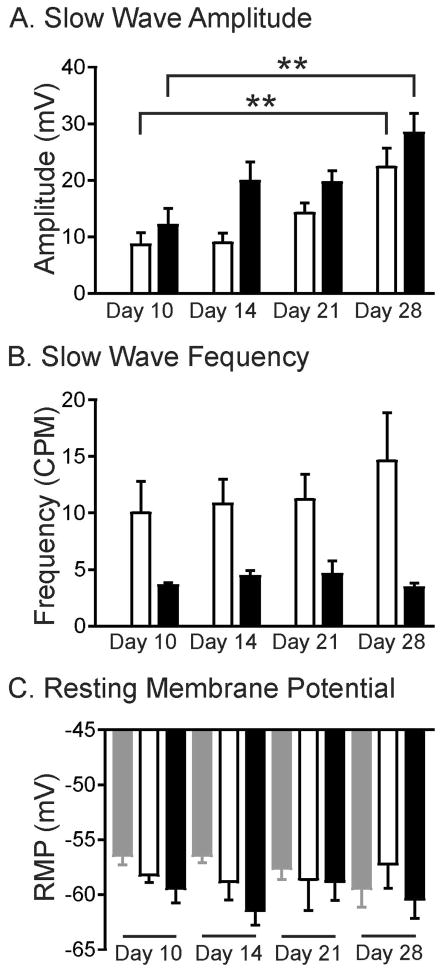
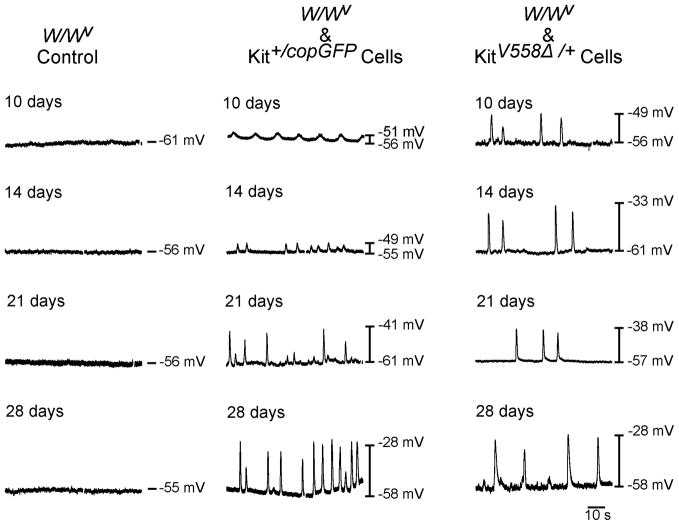
W/WV intestines that were organotypically cultured in the absence of transplanted cells were electrically quiescent, lacking slow waves at all time points investigated (Fig. 7). Resting membrane potentials (RMP) did not change over 28d in culture (i.e. −56.4±0.9 mV at 10d and −59.4±1.7mV at 28d; n=5) and was similar to that observed in native W/WV intestines (Fig. 1B). RMP of W/WV tissues transplanted with Kit+/copGFP derived cells was not significantly different than non-transplanted tissues. However by 10d post-transplantation regular oscillations in membrane potential 8.6±2.1mV in amplitude occurred at a frequency of 10.0±2.8 cycles/min (CPM; n=5; Figs. 6,7A–C). At 28d RMP remained unchanged in cultures (−58.2±0.7mV and −57.2±2.2mV at 10d and 28d respectively; P>0.05) but large amplitude slow waves 22.4±3.31 mV in amplitude occurred at a frequency of 14.6±4.3 CPM (n=5). Slow wave amplitudes were larger at 28d compared to 10d (P=0.0079; Figs. 6,7A).
Figure 7.
Summary of intracellular recordings revealing changes in slow wave amplitude, frequency, but not RMP. (A) Shows time-dependent increases in slow wave amplitude of W/WV cells seeded with Kit+/copGFP (white bars) and KitV558Δ/+ (black bars) cells but not control tissues. (B) Summary of slow wave frequency over time in culture, which tended to be greater in W/WV intestines transplanted with Kit+/copGFP cells than intestines transplanted with KitV558Δ/+ derived cells. (C) RMP was not significantly different in W/WV tissues that were not seeded (grey bars) versus tissues transplanted with Kit+/copGFP (white bars) and KitV558Δ/+ (black bars) derived cells from 10–28d in culture. n=5 for each parameter.
Figure 6.
Development of pacemaker activity in W/WV intestines allotransplanted with cells from intestines of Kit+/copGFP and KitV558Δ/+ mutants. (A) Representative intracellular recordings displaying electrical quiescence in W/WV control non-transplanted tissue cultures (A) at 10,14,21&28d respectively. (B) Development of slow wave activity in W/WV intestines over similar time points following allotransplantation of Kit+/copGFP derived cells. (C) Intracellular recordings from 10–28d demonstrating development of slow waves in a time-dependent manner upon allotransplantation of KitV558Δ/+ derived cells into W/WV intestines.
Allotransplantation of KitV558Δ/+ derived cells led to a similar, time-dependent increase in slow wave amplitude between 10d and 28d in culture. After 10d in culture RMP averaged −59.4±1.4mV and slow waves with amplitudes of 12.1±3 mV occurred at a frequency of 3.6±0.2 CPM (n=5). After 28d in culture RMP remained unchanged (i.e. −60.4±1.7mV) but slow wave amplitude increased to 28.3±3.43 mV, P=0.007 compared to 10d, n=5). Slow wave frequency did not change from 10d to 28d in culture (3.6±0.2 CPM at 10d compared to 3.4±0.4 CPM at 28d; Figs. 6, 7A–C). At each time point investigated, larger amplitude slow waves were observed in KitV558Δ/+ allotransplanted tissues compared to Kit+/copGFP allotransplanted tissues (Fig. 7A). Slow wave activity failed to develop when dispersed cells from either donor were allotransplanted onto the submucosal surface of the circular layer or onto the serosal surface of W/WV intestines (not shown).
DISCUSSION
In the present study we have demonstrated for the first time the feasibility of allotransplantation of ICC into the myenteric region of the small intestine and the establishment of functional pacemaker activity into tissues normally devoid of ICC-MY and slow waves.1, 2 Immunohistochemical analysis was performed to demonstrate the development of Kit+-ICC networks and intracellular microelectrode recordings revealed the development of slow wave pacemaker activity in W/WV small intestines.
It has been previously demonstrated that stem cell factor signaling through the Kit receptor is essential for the development and maintenance of functional ICC networks.33 Stem cell factor or Steel is the natural ligand for Kit35 and disruption in this signaling pathway by use of the neutralizing antibody ACK2 or the receptor antagonist STI-571 led to a loss of ICC and slow wave activity.27–29 Mice with mutations in the Kit receptor (i.e. W/WV) have a disruption in ICC-MY and lack slow wave activity.1, 2 Although Kit signaling is disrupted in W/WV mutants, SCF expression appears normal (Ward and Hwang, unpublished observations). Therefore the disruption in ICC networks and absence of slow waves in the intestines of W/WV mutant mice provides a suitable environment and animal model for allotransplantation studies of ICC into tissues normally devoid of these cells.
We performed allotransplantation of enzymatically-dispersed cells from the tunica muscularis of two different animal models to determine the importance of Kit signaling. In the first model, cells were dispersed from intestines of Kit+/copGFP mice where ICC-MY develop normally.24 Kit+/copGFP mice express green fluorescent protein as a reporter for Kit, allowing us to follow the fate of ICC when allotransplantated onto W/WV host intestines for relatively short periods in culture. Standard immunohistochemical techniques were also used to examine allotransplanted tissues. Using this approach we were able to follow the formation of ICC-networks from single dispersed Kit+ cells.
The second model utilized dispersed cells from KitV558Δ/+ mutant intestines. This mutant was chosen because ICC display a hyperplastic phenotype due to loss of SCF regulation of Kit signaling, leading to the development of GIST.36 When these cells were allotransplanted onto W/WV intestines, Kit+-ICC rapidly became widely distributed but did not form the classical ICC networks observed in intestines of wildtype animals or in organotypically cultured W/WV intestines allotransplanted with Kit+/copGFP dispersed cells (see Fig. 2F). We chose to examine the dispersed cells from the intestines of KitV558Δ/+ mutants because hyperplastic ICC do not develop into stromal tumors in this region of the GI tract. Interestingly although there appeared to be a rapid growth of Kit+-ICC in W/WV intestines allotransplanted with KitV558Δ/+ cells, they did not form discrete networks, however electrical rhythmicity developed in a manner similar to Kit+/copGFP allotransplantations although the frequency of slow waves was less than observed with the Kit+/copGFP transplantations. The difference in slow wave frequencies may be a consequence of the lack of KitV558Δ/+ cells to form networks. In KitV558Δ/+ animals there is a marked hyperplasia of ICC throughout their gastrointestinal tracts where the normal interconnecting networks of ICC are replaced with a dense anastomosing network and ICC interconnections are difficult to resolve. However, the electrical activity remains relatively similar both in amplitude and frequency to that of wildtype controls.37
The mechanisms of maintenance and turnover of ICC, in vivo, remain controversial, however it is well recognized that ICC display a high degree of plasticity and regenerative capacity. ICC restoration has been observed in a variety of models including immunoneutralization,27, 29 partial mechanical obstruction,38 surgical lesion38, 39 and inflammation.40 These investigations showed ICC networks were disrupted and restoration of ICC was observed upon removal of the insult. During normal development of the small intestine, ICC have been shown to incorporate BrdU, suggesting that they undergo cellular division.41 The incorporation of BrdU in ICC decreased with age, but was still observed in mice as old as 24 days, suggesting that division of ICC can occur in mature animals.41–44 When Kit signaling and ICC networks are disrupted in response to a pathophysiological insult, they are capable of repopulating tissues and generating pacemaker activity.27 Although ICC possess a remarkable plasticity, the mechanism of how they repopulate GI tissues is controversial. It has been previously shown that that ICC undergo a phenotypic change and adopt a smooth muscle phenotype, including expression of thick filaments in response to disruption of the Kit signaling pathway.45 Others have recently reported that several cell phenotypes within the tunica muscularis undergo apoptosis including ICC in response to ischemic reperfusion of the intestine and subsequent proliferation was involved in their recovery.46 It has also been suggested that ICC undergo apoptosis in healthy colon as a natural process to regulate numbers that must continually regenerate to maintain intact networks.47
Recently a population of presumed ICC progenitor/stem cells has been identified in murine gastric muscles. These progenitor cells display a KitlowCD44+CD34+Insr+Igf1r+ phenotype and have been postulated to be involved in the constant remodeling of ICC networks.32 Whilst ICC progenitor/stem cells cannot be discounted as a potential source of regeneration, their involvement in the current allotransplant system may be limited. We could not readily identify clusters of KitlowCD44+CD34+cells in the small intestines of wildtype mice, even though large areas of tissues were examined. CD34 was predominantly expressed in smooth muscle cells of the circular and longitudinal layers and in a multi-polar cell population, likely to represent fibroblast-like cells. Further, CD44 was expressed in mature differentiated ICC-MY that formed distinct networks. Finally, when enzymatic dispersions of cells were placed on locations where KitlowCD44+CD34+cells have been reported to be present (other than the myenteric region) they failed to develop into functional networks. This together with the relative rarity of these cells in gastric tissues may preclude their involvement in the restoration of slow waves within the time scale of the organotypic cultures described in the present study. KitlowCD44+CD34+Insr+Igf1r+ progenitors have recently been identified in W/WV gastric tissues,48 however the failure of control W/WV non transplanted tissues to develop ICC (Fig. 2A) or slow wave activity (Fig. 7) suggests that potential endogenous progenitor/stem cells are redundant for the establishment of ICC networks within this allotransplantation system. The possibility exists that donor KitlowCD44+CD34+ Insr+Igf1r+ progenitors may contribute to the establishment of Kit+-ICC in allotransplanted W/WV host intestines. IGF-I was not added to the smooth muscle growth media, fetal bovine serum (FBS) is present at a concentration of 5% and likely contains IGF-I. Supplementation of IGF-I to the organotypic culture media was previously shown to be necessary for ICC progenitor cells to mature and maintain ICC networks in the stomach.32 Therefore, although IGF-I was not added to the smooth muscle culture media in the present study, contributions from this signaling pathway to the establishment of the mature ICC networks in W/WV intestines in the present study cannot be ruled out. Insulin is also present in the smooth muscle growth media (5 μg/ml) used in the present study and is known to activate insulin-like growth factor-1 receptors (Igf1r). The affinity for Igf1r for insulin is reduced compared to Igf1 depending upon the presence of insulin/Igf1 hybrid receptors in tissues, but also cannot be ruled out as a contributor to the proliferation of ICC in the present study.
The use of an allotranplantation approach similar to that described in the present study may provide a valuable means to restore Kit+-ICC into GI tissues from patients naturally devoid of these cells or where ICC numbers have been reduced as a consequence of a pathophysiological insult. Since mature Kit+-ICC possess the capability of mitotic division, small full thickness biopsies could provide sufficient cells from one region of the GI tract for transplantation to another, or from donor to a genetically similar host. This allotransplantation approach may be more successful rather then trying to locate very scare KitlowCD44+CD34+Insr+Igf1r+ progenitor cells within a patient or host for transplantation.
In summary, the development of ICC networks into tissues normally devoid of these cells and the generation of robust slow waves demonstrates for the first time the feasibility of allotransplantation to restore functional pacemaker activity. These findings provide preliminary evidence that cellular transplantation of ICC may provide a means to alleviate gastrointestinal motility disorders in patients that have lost these cells as a consequence of genetic defects, following pathophysiological insult or as a result of natural processes such as aging.
Acknowledgments
This work was supported by NIH DK41315. The Morphology Core Laboratory supported by an equipment grant from the NCRR for the Zeiss LSM510 confocal microscope (1 S10 RR16871) were used for some of the immunohistochemical studies. Part of this work was presented at the Joint Neurogastroenterology and Motility Meeting, Bologna, Italy, 2012 and published in abstract form.49
Footnotes
CONFLICT OF INTEREST
The authors have no competing interests to declare.
AUTHOR CONTRIBUTIONS
CMcC, SJH, EJC and SMW acquired and interpreted data. YB provided technical support. KMS and SMW interpreted results and obtained funding. CMcC, KMS and SMW drafted and critically revised the manuscript.
References
- 1.Huizinga JD, Thuneberg L, Kluppel M, et al. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–9. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 2.Ward SM, Burns AJ, Torihashi S, et al. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480 (Pt 1):91–7. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–43. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 4.Beckett EA, Horiguchi K, Khoyi M, et al. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl(d) mice. J Physiol. 2002;543:871–87. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns AJ, Lomax AE, Torihashi S, et al. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93:12008–13. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward SM, Beckett EA, Wang X, et al. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward SM, McLaren GJ, Sanders KM. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol. 2006;573:147–59. doi: 10.1113/jphysiol.2006.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol. 2003;553:803–18. doi: 10.1113/jphysiol.2003.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cousins HM, Edwards FR, Hickey H, et al. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J Physiol. 2003;550:829–44. doi: 10.1113/jphysiol.2003.042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirst GD, Edwards FR. Generation of slow waves in the antral region of guinea-pig stomach--a stochastic process. J Physiol. 2001;535:165–80. doi: 10.1111/j.1469-7793.2001.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennig GW, Spencer NJ, Jokela-Willis S, et al. ICC-MY coordinate smooth muscle electrical and mechanical activity in the murine small intestine. Neurogastroenterol Motil. 22:e138–51. doi: 10.1111/j.1365-2982.2009.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faussone-Pellegrini MS, Cortesini C. The muscle coat of the lower esophageal sphincter in patients with achalasia and hypertensive sphincter. An electron microscopic study. J Submicrosc Cytol. 1985;17:673–85. [PubMed] [Google Scholar]
- 13.Gockel I, Bohl JR, Eckardt VF, et al. Reduction of interstitial cells of Cajal (ICC) associated with neuronal nitric oxide synthase (n-NOS) in patients with achalasia. Am J Gastroenterol. 2008;103:856–64. doi: 10.1111/j.1572-0241.2007.01667.x. [DOI] [PubMed] [Google Scholar]
- 14.Lyford GL, He CL, Soffer E, et al. Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut. 2002;51:496–501. doi: 10.1136/gut.51.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wedel T, Spiegler J, Soellner S, et al. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology. 2002;123:1459–67. doi: 10.1053/gast.2002.36600. [DOI] [PubMed] [Google Scholar]
- 16.Isozaki K, Hirota S, Miyagawa J, et al. Deficiency of c-kit+ cells in patients with a myopathic form of chronic idiopathic intestinal pseudo-obstruction. Am J Gastroenterol. 1997;92:332–4. [PubMed] [Google Scholar]
- 17.Kenny SE, Vanderwinden JM, Rintala RJ, et al. Delayed maturation of the interstitial cells of Cajal: a new diagnosis for transient neonatal pseudoobstruction. Report of two cases. J Pediatr Surg. 1998;33:94–8. doi: 10.1016/s0022-3468(98)90370-0. [DOI] [PubMed] [Google Scholar]
- 18.Porcher C, Baldo M, Henry M, et al. Deficiency of interstitial cells of Cajal in the small intestine of patients with Crohn’s disease. Am J Gastroenterol. 2002;97:118–25. doi: 10.1111/j.1572-0241.2002.05430.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu G, Qian X, Berezin I, et al. Inflammation modulates in vitro colonic myoelectric and contractile activity and interstitial cells of Cajal. Am J Physiol. 1997;273:G1233–45. doi: 10.1152/ajpgi.1997.273.6.G1233. [DOI] [PubMed] [Google Scholar]
- 20.Rumessen JJ. Ultrastructure of interstitial cells of Cajal at the colonic submuscular border in patients with ulcerative colitis. Gastroenterology. 1996;111:1447–55. doi: 10.1016/s0016-5085(96)70005-7. [DOI] [PubMed] [Google Scholar]
- 21.He CL, Soffer EE, Ferris CD, et al. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–34. doi: 10.1053/gast.2001.26264. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki H, Kajimura M, Osawa S, et al. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006;41:1076–87. doi: 10.1007/s00535-006-1909-8. [DOI] [PubMed] [Google Scholar]
- 23.Ordog T, Takayama I, Cheung WK, et al. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–9. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 24.Ro S, Park C, Jin J, et al. A model to study the phenotypic changes of interstitial cells of Cajal in gastrointestinal diseases. Gastroenterology. 138:1068–78. e1–2. doi: 10.1053/j.gastro.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez-Pinilla PJ, Gibbons SJ, Sarr MG, et al. Changes in interstitial cells of cajal with age in the human stomach and colon. Neurogastroenterol Motil. doi: 10.1111/j.1365-2982.2010.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izbeki F, Asuzu DT, Lorincz A, et al. Loss of Kitlow progenitors, reduced stem cell factor and high oxidative stress underlie gastric dysfunction in progeric mice. J Physiol. 588:3101–17. doi: 10.1113/jphysiol.2010.191023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beckett EA, Ro S, Bayguinov Y, et al. Kit signaling is essential for development and maintenance of interstitial cells of Cajal and electrical rhythmicity in the embryonic gastrointestinal tract. Dev Dyn. 2007;236:60–72. doi: 10.1002/dvdy.20929. [DOI] [PubMed] [Google Scholar]
- 28.Maeda H, Yamagata A, Nishikawa S, et al. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–75. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 29.Torihashi S, Ward SM, Nishikawa S, et al. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- 30.Chabot B, Stephenson DA, Chapman VM, et al. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–9. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 31.Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of cajal from the murine small intestine. J Physiol. 1998;513 (Pt 1):203–13. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorincz A, Redelman D, Horvath VJ, et al. Progenitors of interstitial cells of cajal in the postnatal murine stomach. Gastroenterology. 2008;134:1083–93. doi: 10.1053/j.gastro.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward SM, Burns AJ, Torihashi S, et al. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995;269:C1577–85. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- 34.Vanderwinden JM, Rumessen JJ, De Laet MH, et al. CD34 immunoreactivity and interstitial cells of Cajal in the human and mouse gastrointestinal tract. Cell Tissue Res. 2000;302:145–53. doi: 10.1007/s004410000264. [DOI] [PubMed] [Google Scholar]
- 35.Zsebo KM, Williams DA, Geissler EN, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–24. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 36.Sommer G, Agosti V, Ehlers I, et al. Gastrointestinal stromal tumors in a mouse model by targeted mutation of the Kit receptor tyrosine kinase. Proc Natl Acad Sci USA. 2003;100:6706–11. doi: 10.1073/pnas.1037763100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon JG, Hwang SJ, Hennig GW, et al. Changes in the structure and function of ICC networks in ICC hyperplasia and gastrointestinal stromal tumors. Gastroenterology. 2009;136:630–9. doi: 10.1053/j.gastro.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang IY, Glasgow NJ, Takayama I, et al. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J Physiol. 2001;536:555–68. doi: 10.1111/j.1469-7793.2001.0555c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanagida H, Yanase H, Sanders KM, et al. Intestinal surgical resection disrupts electrical rhythmicity, neural responses, and interstitial cell networks. Gastroenterology. 2004;127:1748–59. doi: 10.1053/j.gastro.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 40.Wang XY, Vannucchi MG, Nieuwmeyer F, et al. Changes in interstitial cells of Cajal at the deep muscular plexus are associated with loss of distention-induced burst-type muscle activity in mice infected by Trichinella spiralis. Am J Pathol. 2005;167:437–53. doi: 10.1016/S0002-9440(10)62988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mei F, Zhu J, Guo S, et al. An age-dependent proliferation is involved in the postnatal development of interstitial cells of Cajal in the small intestine of mice. Histochem Cell Biol. 2009;131:43–53. doi: 10.1007/s00418-008-0515-7. [DOI] [PubMed] [Google Scholar]
- 42.Mei F, Han J, Huang Y, Jiang ZY, Xiong CJ, Zhou DS. Plasticity of interstitial cells of Cajal: a study in the small intestine of adult Guinea pigs. Anat Rec (Hoboken) 2009;292:985–93. doi: 10.1002/ar.20928. [DOI] [PubMed] [Google Scholar]
- 43.Tharayil VS, Wouters MM, Stanich JE, et al. Lack of serotonin 5-HT2B receptor alters proliferation and network volume of interstitial cells of Cajal in vivo. Neurogastroenterol Motil. 2010;22:462–9. doi: 10.1111/j.1365-2982.2009.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanich JE, Gibbons SJ, Eisenman ST, et al. Ano1 as a regulator of proliferation. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1044–51. doi: 10.1152/ajpgi.00196.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torihashi S, Nishi K, Tokutomi Y, et al. Blockade of kit signaling induces transdifferentiation of interstitial cells of Cajal to a smooth muscle phenotype. Gastroenterology. 1999;117:140–8. doi: 10.1016/s0016-5085(99)70560-3. [DOI] [PubMed] [Google Scholar]
- 46.Mei F, Guo S, He YT, et al. Apoptosis of interstitial cells of Cajal, smooth muscle cells, and enteric neurons induced by intestinal ischemia and reperfusion injury in adult guinea pigs. Virchows Arch. 2009;454:401–9. doi: 10.1007/s00428-009-0739-5. [DOI] [PubMed] [Google Scholar]
- 47.Gibbons SJ, De Giorgio R, Pellegrini MS, et al. Apoptotic cell death of human interstitial cells of Cajal. Neurogastroenterol Motil. 2009;21:85–93. doi: 10.1111/j.1365-2982.2008.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bardsley MR, Horvath VJ, Asuzu DT, et al. Kitlow stem cells cause resistance to Kit/platelet-derived growth factor alpha inhibitors in murine gastrointestinal stromal tumors. Gastroenterology. 2010;139:942–52. doi: 10.1053/j.gastro.2010.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ward SM, Mc Cann C, Hwang SJ, Bayguinov Y, Sanders KM. Establishment of pacemaker activity in tissues allotransplanted with interstitial cells of Cajal. Neurogastroenterology and Motility. 2012;24(2) doi: 10.1111/nmo.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]