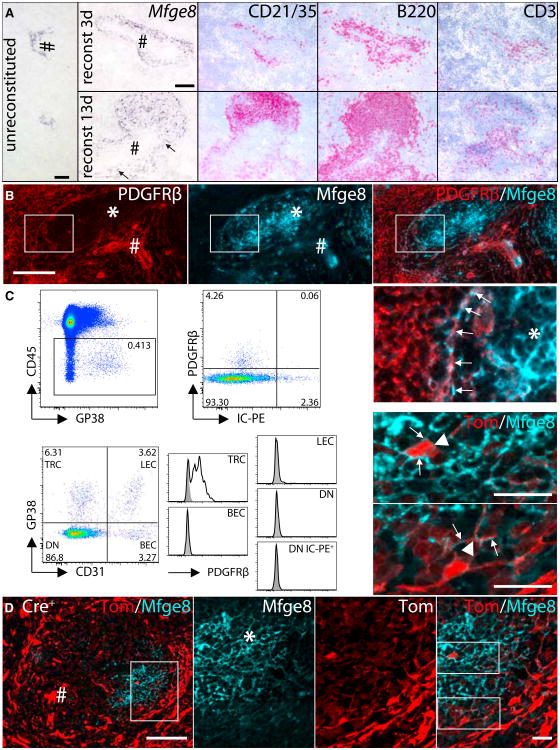
Figure 4. Perivascular Induction of FDC Development.
(A) Rag2−/−γc−/− mice were reconstituted with Mfge8−/− BM and analyzed at 3–13 days (d3–13) after transfer. Mfge8 ISH on unreconstituted Rag2−/−γc−/− spleen (left), d3 (upper row), and d13 (lower row) postreconstitution. Consecutive sections stained for B cells (CD21/35 and B220) and T cells (CD3). Arrows: Mfge8+ cells.
(B) Co-IF of PDGFRβ and Mfge8 on preFDC, but not on mature FDC of WT spleens.
(C) Flow cytometric analysis for PDGFRβ on stromal cells isolated from the inguinal lymph nodes (LN) of mice that had received IC-PE. Within the stromal fraction (CD45−, upper row left) PDGFRβ expression was restricted to the IC-PE negative fraction (upper row right). Expression of PDGFRβ was then determined within the four stromal (CD45−) subpopulations by staining with CD31 and GP38 (lower row left). T zone reticular cells (TRC, CD31−GP38+), lymphatic endothelial cells (LEC, CD31+GP38+), blood endothelial cells (BEC, CD31+GP38−), double-negative (DN) cells (CD31−GP38−) cells containing FDC (DN IC-PE+). Only TRC showed increased expression of PDGFRβ (unshaded) over isotype control stained sample (shaded).
(D) IF staining for Mfge8+ FDC on splenic section from Pdgfrb-Cre+ Ai14+ mice showing strong expression of the reporter protein tdTomato within the vascular components (left, scale bar 20 μm). Higher magnification (indicated by box) on the FDC area (middle (from left to right: Mfge8, tdTomato, overlay, scale bar 20 μm), showing expression of reporter on FDC cell body and dendrites (higher magnification). See also Figure S4.