Figure 2.
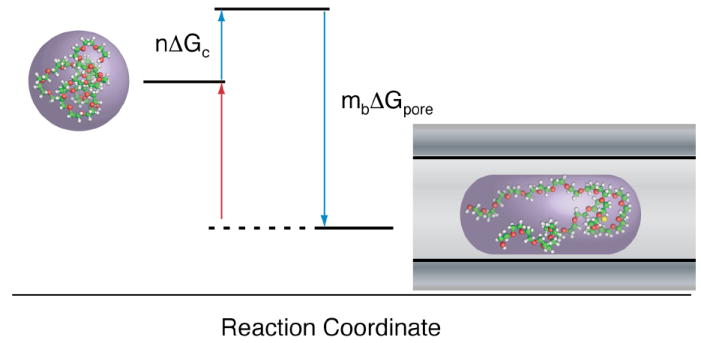
The kinetics of PEG partitioning into the nanopore are described by reversible reactions. PEG kinetics contribute to a description of both the channel conductance and the mean residence time. In order to enter the pore (blue arrows), PEG must overcome an entropic barrier (n ΔGc). The model also accounts for reversible PEG-cation complexes, formed when PEG adsorbs mB cations resulting in a free energy change of mB ΔGpore. In addition to volume exclusion, the adsorbed ions decrease the local ion concentration and further reduce the channel conductance. The mean residence time of PEG is determined by the free energy of dissociation. PEG exits the pore when mB cations dissociate from the complex (red arrow) and follows a single exponential distribution.
