Figure 3.
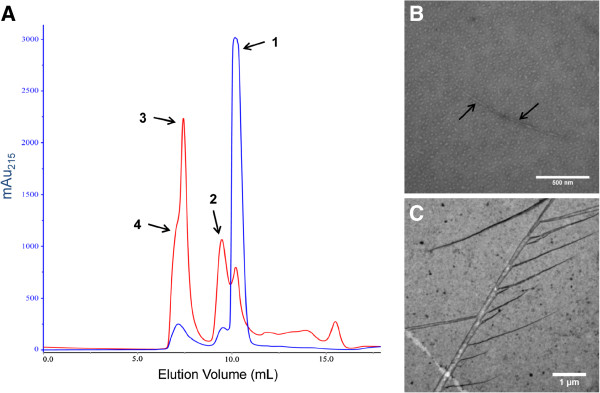
Characterizing the process of ΔK122 PNT oligomerization in solution. (A) SEC analysis (10 nm pore size) of 1 mg·mL-1 ΔK122 before (blue) and after (red) 24 hr incubation with MPD. Peaks 1 through 4 were analyzed with multi-angle light scattering in-line with the SEC (SEC-MALS; Table 1). SEC-MALS data indicate that ΔK122 exists in a monomer/dimer equilibrium (Peak 1) and that upon incubation with MPD, higher molecular weight species form, corresponding to 14, 20 and 40 pilin monomers for Peaks 2, 3 and 4, respectively (Table 1). Aliquots of SEC-separated ΔK122 (15 mg·mL-1) incubated with MPD for 24 hr corresponding to Peaks 1/2 and 3/4 were negatively stained with 4% uranyl acetate and visualized with TEM. (B) The ΔK122 monomer/dimers in Peaks 1/2 are seen as aggregates in TEM, and form pilin fibrils (highlighted by arrows) upon addition of the oligomerization initiator MPD to the protein solution. (C) Pilin fibrils associate into PNTs (Peaks 3/4), which can then coalesce into larger PNT bundles that show similar structures as those formed when C11-SH is used as the inducer of pilin oligomerization [23,87].
