Abstract
Background
The number of unicompartmental knee arthroplasties (UKAs) is growing worldwide. Because lateral UKAs are performed much less frequently than medial UKAs, the limited information leaves unclear whether UKAs have comparable survival and health-related quality of life (HRQoL) of the lateral UKA to medial UKAs.
Questions/purposes
We therefore compared the (1) survivorship and (2) HRQoL after lateral versus medial cemented mobile-bearing UKAs and (3) determined whether there is an association of survival to modifications of surgical technique in one of three phases.
Methods
We retrospectively reviewed 558 patients who underwent mobile-bearing UKAs from 2002 to 2009. From the records we determined revision of the joint for any reason and revision for aseptic loosening. Patients reported their physical function, pain, and stiffness as measured by the WOMAC, SF-36 physical-component summary (PCS), and Lequesne knee score. Information regarding implant survival was collected for 93% of the patients. We analyzed the patients separately by three phases based on surgical changes associated with each phase (1: initial technique; 2: improved cementing; 3: additional bone resection to ensure backward sliding of the inlay without impingement). The minimum followup was 2.1 years (mean, 6 years; range, 2.1–9.8 years).
Results
Implant survival was 88% at 9 years. We found similar implant survival rates for medial (90%) and lateral UKAs (83%). In all HRQoL measures, patients receiving a medial UKA had better mean scores compared with patients who had a lateral UKA: WOMAC physical function (23 versus 34, respectively) and pain (21 versus 34) and SF-36 PCS (41 versus 38). There were no survival differences by surgical phase.
Conclusions
Our observations suggest a medial UKA is associated with superior HRQoL when compared with a lateral UKA, although implant survival is similar.
Level of Evidence
Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
With more than 600,000 procedures in the United States each year, knee arthroplasty is one of the most common major surgical procedures [9]. For patients with unicompartmental osteoarthritis of the knee, a unicompartmental knee arthroplasty (UKA) frequently is used as an alternative to a TKA with increased use reported by the National Joint Registry for England and Wales (from 8.0% in 2003 to 8.4% in 2011) [28] and a current frequency of 9.7% reported by the Australian joint registry [8].
UKAs can be performed on the medial or lateral side. A lateral UKA reportedly is performed approximately 10 times less frequently than a medial UKA [35] and therefore there is limited literature regarding the outcome of lateral UKAs. In a recent review [18] of lateral UKAs, nine retrospective studies were identified, of which four involved fewer than 20 patients; 83 patients were analyzed in the largest study [6]. In that study, the cumulative survival rates for a lateral UKA were 83% at 10 years and 74% at 15 years. Some studies [17, 36, 40] analyzed lateral and medial UKAs with conflicting findings regarding risk of revisions. For example, in the earliest study [36], there was a survival of 83% at 3½ years for the lateral and 99% for the medial UKA. In the most recent study [40], there were no revisions in 31 lateral UKAs, but six revisions in 147 medial UKAs, whereas another study [17] reported similar revision rates for medial and lateral UKAs. Of the studies evaluating health-related quality of life (HRQoL) outcomes such as the WOMAC™ [40], the Hospital for Special Surgery Score [30, 32], or The Knee Society Score© [17, 23, 24], only one study [40] compared one of these HRQoL outcomes in medial versus lateral UKAs. That study did not describe a difference between medial and lateral UKAs regarding the specific HRQoL outcome (WOMAC).
Several prostheses are available for a UKA [28]. König et al. [19] reported one mobile-bearing implant was associated with lower blood loss, better pain relief, and quicker rehabilitation than after TKA. They also reported the implantation required a “sound operative technique” to avoid complications [19]. A revision rate of 21% has been reported at a mean of 22 months after implantation of 43 of these same mobile-bearing UKAs [4]. According to data from the Australian Arthroplasty Registry [7], this particular device (Preservation® UKA; DePuy International Ltd, Leeds, UK) had a high revision rate owing to the mobile-bearing tibial component (mobile bearing, 4.4 revisions/100 observed years; fixed bearing, 2.2 revisions/100 observed years; all types of primary UKAs, 1.9 revisions/100 observed years). Although the revision rate for the Preservation® fixed bearing was calculated based on 2087 implants, there were only 401 Preservation® mobile bearings in the registry. This reported high revision rate for the Preservation® mobile bearing contrasts with our clinical experience; the senior author (WH) implanted more than 500 Preservation® mobile-bearing prostheses during a 7-year period.
The current literature does not adequately inform the surgeon what to expect if a lateral UKA is performed. Apart from technical considerations, this could be why a lateral UKA is performed approximately 10 times less frequently than a medial UKA. Because most orthopaedic surgeons are familiar with the clinical performance of a medial UKA, we intended to describe the clinical performance of a lateral UKA in comparison to a medial UKA.
We therefore compared the (1) survivorship and (2) HRQoL after lateral versus medial cemented mobile-bearing UKAs and (3) determined whether there is an association of implant survival and surgical phases.
Patients and Methods
We retrospectively reviewed all 558 patients who underwent a UKA with implantation of a Preservation® mobile-bearing prosthesis for medial or lateral arthritis of the knee on at least one knee from April 2002 to October 2009. During that same time, we treated 656 patients with TKAs. If patients had bilateral surgery, only the first surgery was considered for further evaluation [1]. We routinely used no other type of prosthesis during this time. The indications for UKA were (1) disabling knee pain; (2) unicompartmental tibiofemoral disease with full-thickness cartilage in the other compartment; (3) fixed flexion deformity of 15° or less; or (4) a passively correctable valgus [14] or varus deformity less than 15°. The contraindications were (1) loss of joint space in the patellofemoral joint of over 50%; and (2) a concave aspect of the patella formed by osteophytes on the skyline view. Five hundred twenty-five of the 558 patients (94%) were treated for primary osteoarthritis, 26 for osteonecrosis/Ahlbäcks disease, four for posttraumatic arthritis, and three for rheumatoid arthritis. Although 430 patients (77%) underwent a medial UKA, 128 (23%) had a lateral UKA. The mean age of the patients at the time of surgery was 73.6 years (range, 44–91 years), their mean weight was 79.4 kg (range, 40–166 kg), and 67% of the patients were women. Minimum followup was 2.1 years (mean, 6.0 years; range, 2.1–9.8 years). No patients were recalled specifically for this study; all data were obtained from medical records and a questionnaire.
The Preservation® UKA system offers fixed and mobile-bearing designs, of which we exclusively used the mobile-bearing design. The femoral component has a J-curve in the sagittal plane, and the tibial component has a curved bearing track that semiconstrains the congruent polyethylene meniscal bearing with its concavity toward the intercondylar eminence. Therefore, it can glide in the AP direction but not in the mediolateral direction (Fig. 1).
Fig. 1.
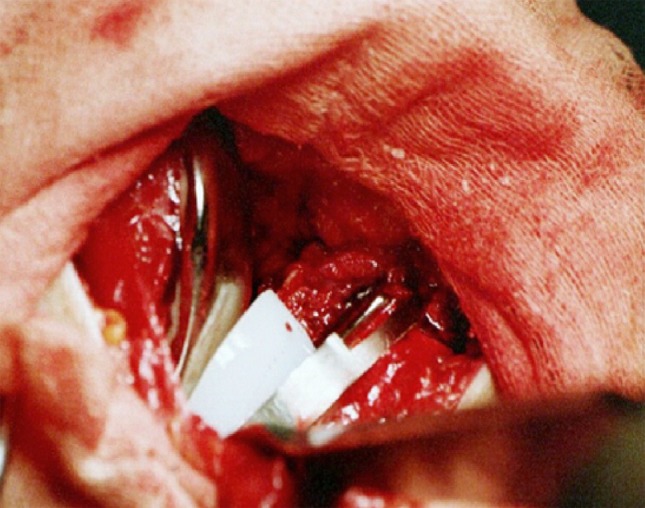
An intraoperative photograph shows the track for the mobile-bearing prosthesis in the UKA.
One of us (WH) performed all the surgeries. For a medial UKA, an anteromedial approach was used (Fig. 2), and for lateral UKA, an anterolateral approach was used. The incision reached from the tibial tubercle to the insertion of the mediolateral vastus muscle into the quadriceps tendon. Preparation was continued below the mediolateral vastus muscle to separate the joint capsule up into the superior recesses. The patella was subluxated to the nonaffected side. In lateral osteoarthritis, we aimed to reconstruct the physiologic axis [14]. For patients with medial osteoarthritis, we aimed to restore the presumed prepathologic varus alignment (compared with the contralateral side), avoiding overcorrection of the varus deformity [14, 39]. During surgery, the ACL was not present or sufficient in 18 patients (2.1%).
Fig. 2A–B.
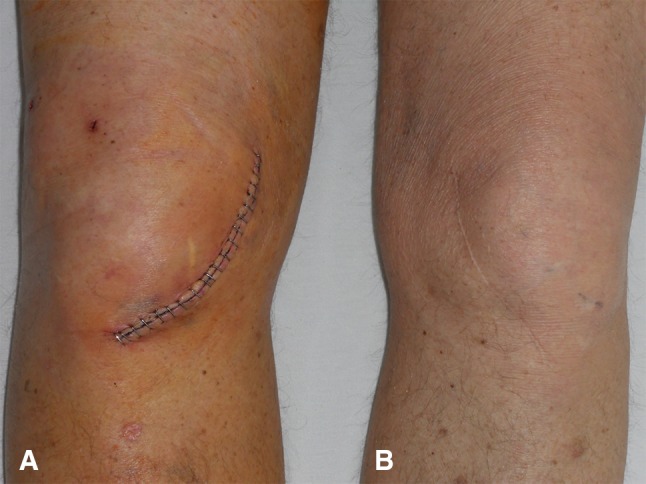
A clinical picture shows the minimally invasive anteromedial approach (A) at Postoperative Day 5 and (B) at 2 years.
There were three phases of surgical technique. In Phase I, we used the original technique recommended by the manufacturer. Beginning in August 2002 (Phase II), we modified the technique by manually enlarging the tibial keel groove after the keel osteotome has been used to increase the width of the cement-mantle for the keel, lavaging and drying of the bone surface while using a tourniquet at 350 mmHg, and applying additional cement into the fin and drill holes. The components were cemented in sequential steps with the femoral component being cemented only after a trial reposition was performed after polymerization of the cement of the tibial component. When cementing the femoral component, the dorsal aspect of the femoral implant was pushed parallel along the resected bone surface. Special attention was given beforehand when performing the femoral cuts to ensure good bone-cement-implant contact, avoiding liftoff. Starting in June 2003 (Phase III), in addition to the regular L-cut for the proximal tibial resection, we made a second L-cut at an angle of 30° in the region of the PCL insertion close to the intercondylar eminence to achieve free backward movement of the mobile insert during flexion. Lateral and medial UKAs were performed in all phases of surgical technique and there was no difference regarding the proportion of medial versus lateral UKA by phase of surgical technique (chi square = 3.221; p = 0.200).
After surgery, patients participated in a standard postoperative program of initially daily individual physiotherapy, consisting of mobilization starting on the first postoperative day using a walker or crutches, ROM activities, exercises for improvement of muscle tension, venous return, balance, coordination and gait, and instruction in activities of daily living, including transfers, walking, and negotiation of stairs and uneven surfaces. This program was continued for a period of approximately 5 weeks. Continuous passive motion machines were used on a daily basis after removal of suction drains for approximately 2 weeks.
At discharge from the hospital, patients were invited to return for followup at 6 to 8 weeks after surgery, at which time a history and clinical evaluation was performed. Further diagnostic tests were performed if patients were not satisfied or if the physical examination was not within expected limits. Return to work was not recommended before 6 to 8 weeks. Impact sports activities were usually allowed 6 months after surgery.
Complications during surgery included a rupture of the ACL in two patients toward the end of surgery, the patella fractured in one patient, and there were cancellous fractures at the dorsal aspect of the condyle in one patient. There were no infections.
Starting in December 2011, all patients were sent a questionnaire and a request for written consent to participate in the study. The questionnaire consisted of items regarding possible revision surgery, including date and name of hospital performing the revision, the SF-36, WOMAC, Lequesne score, comorbidities, EQ-5D, current medications, demographics, and patient satisfaction. We assessed implant survival by patients’ responses from the questionnaire, which were crosschecked by operative notes, with revision for any reason, aseptic failure, and progression of the osteoarthritis to other compartments of the knee as end points. Further outcomes included HRQoL, as measured by self-reported physical function with the WOMAC™ [10] in a validated translated version [37] and two additional domains, pain and stiffness. Responses were recorded on a visual analog scale with terminal descriptors. Scores were added for each category and standardized to a score of 0 to 100 with higher scores indicating less physical function, more pain, and more stiffness. The WOMAC™ is recommended as a suitable outcome for this setting [29]. We also evaluated the patients using the Lequesne knee score [20, 22] and the physical component summary (PCS) of the SF-36, both in a validated translated version [11, 38].
If a questionnaire was returned because it could not be delivered, the last known treating physician or the registration of address office was contacted to identify the current address. If patients did not respond, the questionnaire was sent as many as three more times. Patients still not responding were contacted by telephone to determine the reason for nonresponse and were asked whether they had additional surgery on the surgically treated knee and their satisfaction. Of the 558 patients, 383 returned a questionnaire, representing 79.5% of the patients still alive. Of the 175 who did not respond, we determined the reasons for lack of response for 154 (Table 1). We obtained information on the status of the UKAs in 133 of these patients. Of the 76 patients who died, we were able to identify the status of the knee at the time of death by contacting the last general physician, orthopaedic surgeon, or the relatives of 56 patients. For the remaining 20 patients who had died, we either were unable to identify relatives or physicians treating the patients up to their death or the last status of the knee before the patient’s death was unknown. In the remaining 21 of the 175 patients, we were unable to contact the patient or relatives, and the physicians were out of practice, did not have a current patient address, or were not able to provide current data regarding the patient’s knee. These patients were considered lost to followup.
Table 1.
Reasons for not returning questionnaires
| Reason | Count |
|---|---|
| Died | 76 |
| Had revision surgery, questionnaire would not reflect the UKA | 6 |
| Revision surgery has been suggested, but he or she does not want it | 2 |
| Revision surgery is planned | 1 |
| Dementia/cerebrovascular accident | 16 |
| Has had several other, nonknee-related surgeries or comorbidities | 14 |
| States that he or she has already filled in/wants to fill in questionnaire | 11 |
| Eye problems, cannot read questions | 3 |
| Too many questions | 3 |
| Out of the country (Canada, Bosnia-Herzegovina) | 2 |
| Did not have time, relative died recently | 2 |
| Did not have time, has been in hospital | 2 |
| Does not want to be bothered by questionnaire | 2 |
| No reason given | 2 |
| Does not want to sign informed consent | 2 |
| Feels too old | 2 |
| Did not have time, husband critically ill | 1 |
| Refuses, annoyed by behavior of hospital staff at followup examination | 1 |
| Is not satisfied | 1 |
| Is skeptical about the transfer of personal data | 1 |
| Has been on vacation | 1 |
| Wants to wait to fill in questionnaire until nonknee-related surgery has been performed | 1 |
| Hung up phone | 1 |
| Surgery is too long ago | 1 |
| Total | 154 |
UKA = unicompartmental knee arthroplasty.
We calculated the survival time of the implant as the time between the day of followup or revision and the day of surgery. Survivorship analysis was performed using the Kaplan-Meier method. We also simulated a worst case scenario, assuming that all patients lost to followup underwent revision surgery. To adjust for potential confounding variables, a multivariate Cox proportional hazards model with revision for any reason as the end point was developed in which we examined the association with lateral versus medial UKA while controlling for sex, age, weight, surgical phase, and the diagnosis of osteonecrosis. HRQoL data were tested for normal distribution with the Kolmogorov-Smirnov test. To compare the results of lateral with medial UKA, we used the Mann-Whitney U test. Effect size d [2], the standardized differences between two groups, was calculated as described by Cohen [12]. All p values are two-tailed; no corrections were made for multiple comparisons.
Results
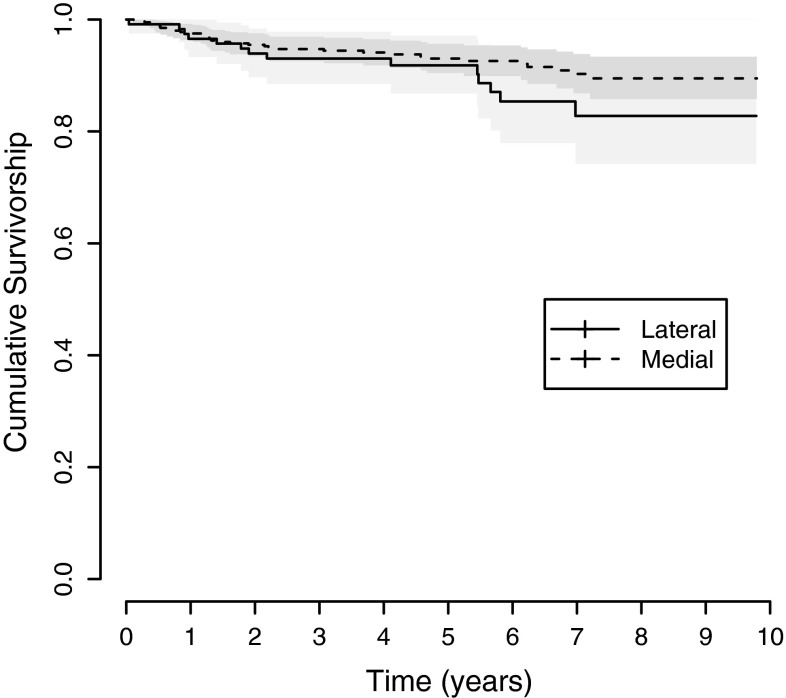
Overall implant survival was 88% at 9 years. Forty-six patients had revision surgery for any reason (Table 2). There was one case of failure of the femoral component, and there were no infections. We observed 2828 component years, resulting in 1.63 revisions per 100 observed component years for revisions for any reason and 1.10 revisions per 100 observed component years for revisions for aseptic loosening, including revisions for unknown causes (Table 3). When stratifying implant survival by medial and lateral compartments, there were similar values for implant survival for medial (89.5% [95% CI, 85.8%–93.4%]) compared with lateral UKA (82.8% [95% CI, 74.2%–92.3%]) (Table 4). The Kaplan-Meier Curve suggests that the survival is almost identical up to the fifth postoperative year, after which the lateral UKAs appear to perform worse; however, that observation was not significant (Fig. 3). When simultaneously assessing the association between revision for any reason and medial versus lateral UKA, while controlling for sex, age, weight, surgical phase, and the diagnosis of osteonecrosis, the probability of implant revision was similar for lateral and medial UKAs (Table 5). When running a worst-case scenario, assuming that all patients lost to followup had revision surgery resulted in a survival of 80% (95% CI, 74%–86%) for the whole cohort at 9 years, with 83% (95% CI, 77%–89%) survival for the medial and 70% (95% CI, 58%–85%) survival for the lateral UKA.
Table 2.
Reasons for revision surgery
| Reason | Medial UKA | Lateral UKA | Total |
|---|---|---|---|
| Aseptic loosening | 16 | 6 | 22 |
| Progression of arthritis to contralateral compartment | 5 | 2 | 7 |
| Fracture | 2 | 2 | 4 |
| Progression of arthritis and aseptic loosening tibial component | 2 | 0 | 2 |
| Progression of arthritis and impression of patella by lateral tibial component | 0 | 1 | 1 |
| Arthroscopy, without change of components | 0 | 1 | 1 |
| Internal fixation with screws, no change of implant components | 0 | 1 | 1 |
| Impingement | 0 | 1 | 1 |
| Unknown | 7 | 0 | 7 |
| Total | 32 | 14 | 46 |
UKA = unicompartmental knee arthroplasty.
Table 3.
Observed component years by revision type
| Variable | Revision for any reason | Revision for aseptic failure or unknown reason | Revision for progression of osteoarthritis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Medial UKA | Lateral UKA | Total | Medial UKA | Lateral UKA | Total | Medial UKA | Lateral UKA | Total | |
| Observed component years in patients who did not have revision surgery | 2130 | 572 | 2702 | 2159 | 597 | 2756 | 2180 | 602 | 2782 |
| Observed component years in patients until revision | 82 | 43 | 125 | 53 | 19 | 72 | 32 | 14 | 45 |
| Total observed component years | 2212 | 616 | 2828 | 2212 | 616 | 2828 | 2212 | 616 | 2828 |
| Number of revisions | 32 | 14 | 46 | 25 | 6 | 31 | 7 | 3 | 10 |
| Number of revisions per 100 observed component years | 1.45 | 2.27 | 1.63 | 1.13 | 0.97 | 1.10 | 0.32 | 0.49 | 0.35 |
UKA = unicompartmental knee arthroplasty.
Table 4.
Life tables for medial and lateral UKAs and for the whole cohort
| Time (years) | Medial UKA | Lateral UKA | Whole cohort | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients at risk | Number of events | Survival (%) | Lower 95% CI | Upper 95% CI | Number of patients at risk | Number of events | Survival (%) | Lower 95% CI | Upper 95% CI | Number of patients at risk | Number of events | Survival (%) | Lower 95% CI | Upper 95 CI | |
| 0 | 401 | 0 | 100.0 | 100.0 | 100.0 | 117 | 0 | 100.0 | 100.0 | 100.0 | 518 | 0 | 100.0 | 100.0 | 100.0 |
| 1 | 390 | 9 | 97.8 | 96.3 | 99.2 | 112 | 4 | 96.6 | 93.3 | 99.9 | 502 | 13 | 97.5 | 96.1 | 98.8 |
| 2 | 375 | 9 | 95.5 | 93.5 | 97.5 | 106 | 3 | 93.9 | 89.7 | 98.4 | 481 | 12 | 95.1 | 93.3 | 97.0 |
| 3 | 331 | 3 | 94.7 | 92.5 | 96.9 | 95 | 1 | 93.0 | 88.5 | 97.8 | 426 | 4 | 94.3 | 92.4 | 96.4 |
| 4 | 278 | 2 | 94.1 | 91.8 | 96.5 | 77 | 0 | 93.0 | 88.5 | 97.8 | 355 | 2 | 93.9 | 91.8 | 96.0 |
| 5 | 235 | 3 | 93.0 | 90.4 | 95.7 | 66 | 1 | 91.8 | 86.8 | 97.1 | 301 | 4 | 92.8 | 90.4 | 95.1 |
| 6 | 180 | 1 | 92.6 | 89.9 | 95.4 | 45 | 4 | 85.4 | 77.9 | 93.5 | 225 | 5 | 91.0 | 88.3 | 93.8 |
| 7 | 135 | 4 | 90.3 | 86.8 | 93.8 | 31 | 1 | 82.8 | 74.2 | 92.3 | 166 | 5 | 88.6 | 85.3 | 92.1 |
| 8 | 68 | 1 | 89.5 | 85.8 | 93.4 | 18 | 0 | 82.8 | 74.2 | 92.3 | 86 | 1 | 88.0 | 84.5 | 91.7 |
| 9 | 25 | 0 | 89.5 | 85.8 | 93.4 | 8 | 0 | 82.8 | 74.2 | 92.3 | 33 | 0 | 88.0 | 84.5 | 91.7 |
UKA = unicompartmental knee arthroplasty; CI = confidence interval.
Fig. 3.
Kaplan-Meier survival curves for the Preservation® mobile-bearing prosthesis used in the UKAs by compartment (medial, lateral) are shown. Implant survival up to Postoperative Year 5 was 93.0% for medial UKAs and 91.8% for lateral UKAs. However, beginning with Postoperative Year 6, implant survival was 92.6% for medial UKAs and 85.4% for lateral UKAs. The shading indicates the 95% CI.
Table 5.
Cox regression model with revision for any reason as the end point
| Variable | Exp(B) | Lower 95% CI | Upper 95% CI | p value |
|---|---|---|---|---|
| Lateral versus medial UKA | 1.376 | 0.58 | 3.25 | 0.466 |
| Female versus male | 1.633 | 0.67 | 3.95 | 0.277 |
| Age | 1.014 | 0.96 | 1.07 | 0.606 |
| Weight | 1.010 | 0.98 | 1.04 | 0.466 |
| Diagnosis of osteonecrosis versus other diagnosis | 1.446 | 0.33 | 6.33 | 0.625 |
| Surgical phase | 0.385 | |||
| Phase I | 2.263 | 0.52 | 9.78 | 0.274 |
| Phase II | 0.640 | 0.19 | 2.18 | 0.475 |
CI = confidence interval; UKA = unicompartmental knee arthroplasty.
In all health-related outcomes, there were lower mean measures of HRQoL in patients receiving a lateral UKA when compared with patients receiving a medial UKA: WOMAC physical function (34 versus 23; p = 0.030) and pain (34 versus 21; p = 0.003) and SF-36 PCS (38 versus 41; p = 0.044) (Table 6).
Table 6.
Health-related quality of life at followup
| Scoring system | Whole cohort | Medial UKA | Lateral UKA | p value* | Effect size d† | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | SE | Mean ± SD | SE | Mean ± SD | SE | |||
| WOMAC™ function score (points)‡ | 25.1 ± 25.5 | 1.5 | 23.4 ± 24.3 | 1.5 | 33.6 ± 29.5 | 4.1 | 0.030 | 0.38 |
| WOMAC™ pain score (points)‡ | 22.3 ± 27.4 | 1.6 | 21.3 ± 26.5 | 1.6 | 33.7 ± 31.3 | 3.9 | 0.003 | 0.43 |
| WOMAC™ stiffness score (points)‡ | 21.4 ± 26.7 | 1.6 | 21.9 ± 27.1 | 1.6 | 22.8 ± 27.1 | 3.6 | 0.962 | 0.04 |
| SF-36 PCS (points)§ | 40.8 ± 10.5 | 0.6 | 41.4 ± 10.2 | 0.6 | 38.0 ± 11.4 | 1.5 | 0.044 | 0.32 |
| SF-36 MCS (points)§ | 49.2 ± 10.8 | 0.6 | 49.5 ± 10.5 | 0.6 | 48.1 ± 12.2 | 1.6 | 0.714 | 0.12 |
| Lequesne knee score (points)‖ | 7.0 ± 3.8 | 0.2 | 6.8 ± 3.7 | 0.2 | 7.9 ± 4.1 | 0.5 | 0.089 | 0.26 |
* All p values are based on the Mann-Whitney U-test; †effect size d is the difference between the means divided by the pooled SD; ‡scores range from 0 to 100 with lower scores representing better quality of life; §higher scores represent better quality of life; ‖lower scores represent better quality of life; UKA = unicompartmental knee arthroplasty; PCS = physical component summary; MCS = mental component summary.
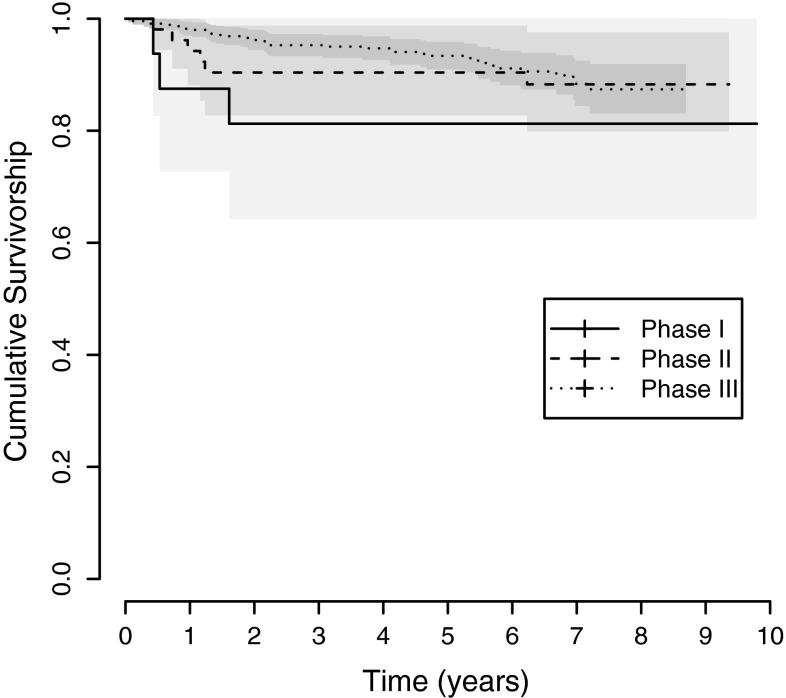
An association between survival and surgical phase could be identified with the earliest surgical phase associated with earlier failure. The 9-year survival was 81.2% (95% CI, 64%–100%) for Phase I and 88.3% (95% CI, 80%–98%) for Phase II compared with the 8-year survival of 87.4% (95% CI, 83%–92%) for Phase III (Table 7). These differences were most pronounced when comparing Phase I with II. However, the survival difference between Phase II and III was evident only before Postoperative Year 5 (Fig. 4).
Table 7.
Life tables by phase of surgical technique
| Time (years) | Phase I | Phase II | Phase III | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients at risk | Number of events | Survival | Lower 95% CI | Upper 95% CI | Number at risk | Number of events | Survival | Lower 95% CI | Upper 95% CI | Number at risk | Number of events | Survival | Lower 95% CI | Upper 95% CI | |
| 0 | 16 | 0 | 100.0% | 100.0% | 100.0% | 52 | 0 | 100.0% | 100.0% | 100.0% | 450 | 0 | 100.0% | 100.0% | 100.0% |
| 1 | 14 | 2 | 87.5% | 72.7% | 100.0% | 49 | 3 | 94.2% | 88.1% | 100.0% | 439 | 8 | 98.2% | 97.0% | 99.4% |
| 2 | 13 | 1 | 81.2% | 64.2% | 100.0% | 45 | 2 | 90.4% | 82.7% | 98.8% | 423 | 9 | 96.2% | 94.4% | 98.0% |
| 3 | 13 | 0 | 81.2% | 64.2% | 100.0% | 44 | 0 | 90.4% | 82.7% | 98.8% | 369 | 4 | 95.3% | 93.3% | 97.3% |
| 4 | 12 | 0 | 81.2% | 64.2% | 100.0% | 43 | 0 | 90.4% | 82.7% | 98.8% | 300 | 2 | 94.7% | 92.6% | 96.8% |
| 5 | 12 | 0 | 81.2% | 64.2% | 100.0% | 43 | 0 | 90.4% | 82.7% | 98.8% | 246 | 4 | 93.4% | 90.9% | 95.9% |
| 6 | 12 | 0 | 81.2% | 64.2% | 100.0% | 43 | 0 | 90.4% | 82.7% | 98.8% | 170 | 5 | 91.1% | 88.1% | 94.3% |
| 7 | 12 | 0 | 81.2% | 64.2% | 100.0% | 42 | 1 | 88.3% | 79.9% | 97.6% | 112 | 4 | 88.4% | 84.4% | 92.5% |
| 8 | 12 | 0 | 81.2% | 64.2% | 100.0% | 42 | 0 | 88.3% | 79.9% | 97.6% | 32 | 1 | 87.4% | 83.1% | 91.9% |
| 9 | 12 | 0 | 81.2% | 64.2% | 100.0% | 21 | 0 | 88.3% | 79.9% | 97.6% | |||||
CI = confidence interval.
Fig. 4.
Kaplan-Meier survival curves for the Preservation® mobile-bearing prosthesis used in the UKAs by surgical technique phase (Phases I–III) are shown. The 9-year survival was 81.2% for Phase I and 88.3% for Phase II compared with the 8-year survival of 87.4% for Phase III. The shading indicates the 95% CI.
Discussion
The rate of UKAs is growing rapidly with only limited information regarding lateral UKAs compared with medial UKAs. That information, however, is crucial, because currently only 10% of all UKAs are performed on the lateral side. Ours is the largest study comparing the results of medial with lateral UKA and is one of the few studies [40] evaluating measures of HRQoL in addition to implant survival in this growing patient group. The dimensions of HRQoL such as physical function, pain, and joint stiffness are recommended as a rationale for implementation of the best standard of care [13]. We compared the (1) survivorship and (2) HRQoL after lateral versus medial cemented mobile-bearing UKAs and (3) determined whether there is an association of the surgical phases on implant survival.
Our results should be interpreted in view of several limitations. First, as is common in retrospective studies, we had no information regarding the HRQoL at the time of surgery; therefore, the groups could be biased. However, because we used the same indication criteria for UKA in the whole cohort, and we report on all patients who received a UKA during the study period, we believe we have a homogeneous cohort and therefore it is reasonable to compare medial versus lateral UKAs. Second, although we achieved a followup of 93% regarding implant survival, 41 patients died or could not be contacted. It is possible the rate of revision in these patients is greater than in the patients who could be contacted. However, because the majority of patients who had revision surgery underwent that surgery in our center, we believe this would not substantially bias our cohort.. The worst-case scenario also showed no differences between survival of medial and lateral UKAs. Third, these results reflect one implant and one surgeon. Therefore, the external validity of the study is limited. However, the HRQoL outcomes in our study compare well with those of other studies after UKA [15, 21, 25]. Therefore, it appears reasonable to assume the results of our large cohort could be transferable to some extent to the population having UKAs. Fourth, as is common in studies evaluating HRQoL, there is no radiographic assessment at followup. As such, there could be other knees where loosening is evident but that may not have been scheduled for surgery. Even registries do not analyze loosening but use revision as the end point. Fifth, for the assessment of HRQoL, we could contact only 79.5% of all patients still alive. However, this is only slightly less than the benchmark of 80% [33] described for this purpose. Sixth, our findings with this particular device may not be applicable to other devices, whether fixed or mobile bearing.
We determined 1.63 revisions per 100 observed component years. This compares well with the performance of other implants, where 1.9 revisions per 100 observed years have been reported in the Australian Joint Replacement Registry [7] as the average for all types of primary UKA. If only revisions for aseptic loosening were considered (including unknown reasons), the revision rate was even lower (1.10; Table 3). It is unclear why the Australian registry and other studies have reported higher revision rates for the particular implant used in this study. All other studies reporting on the implant have considerably fewer patients, and even the Australian registry observed fewer patients and approximately 1000 fewer component years than our cohort (1847 observed component years in the Australian registry versus 2828 in our study). If the phases of surgical technique were considered a learning curve, it could be hypothesized only a few of the other authors or contributors to the Australian registry have passed the learning curve. In addition, this implant was one of the first devices to be included from introduction in the Australian registry. This could mean the learning curve was captured more for this implant than perhaps for other implants included in the registry that would have been implanted before that registry started. Unfortunately, there is no information in the registry regarding the number of surgeons who have used the implant nor is there information regarding the number of implants that have been used at specific hospitals. If that information were available, one could calculate the prior experience of surgeons with specific implants, which would help in identifying learning curves of implants. Therefore, it is suggested joint arthroplasty registries should calculate such an index in the future, because it might influence implant survival, possibly even to a greater extent than some other parameters currently being studied in the registries.
In contrast to another report regarding the Preservation® UKA that had a failure rate of 38% of the femoral component after 1 year when an all-polyethylene tibial component was used in 36 UKAs [26], we observed only one case of failure of the femoral component. The reasons for this difference are unknown. Apart from design issues, it could be hypothesized these differences are related to the cementing technique. Although some surgeons cement the tibial and femoral components at the same time, we always cemented the tibial component first. After polymerization, we performed a trial reposition and cemented the femoral component at a second step.
In our study, patients with medial UKAs had a better HRQoL compared with patients with lateral UKAs. This finding of a better HRQoL after medial versus lateral UKA has not been described before, mostly because measures of HRQoL were not analyzed or the numbers of patients were too small [5, 6, 16, 18, 23, 24, 27, 30–32, 34, 36, 40]. The question remains whether the inferior results of lateral UKA are attributable to the surgical procedure or to the disease, lateral osteoarthritis. If they are attributable to the disease, one might expect that patients with lateral osteoarthritis also would have inferior results if they undergo a TKA. Unfortunately, we were unable to identify any published studies analyzing the effect of a preoperative valgus versus varus deformity on the HRQoL after TKA. We could identify only survival data of lateral versus medial UKA (Table 8).
Table 8.
Outcomes of lateral versus medial UKA in the literature
| Study | Year | Number of patients | Number of lateral UKAs | Number of medial UKAs | Variables reported | Lateral UKA | Medial UKA | Difference lateral versus medial | Followup (years) | Implant details |
|---|---|---|---|---|---|---|---|---|---|---|
| Altuntas et al. [3] | 2012 | 58 | 64 | 0 | Survivorship | 4 knees had revision surgery for any reason, two of which were not prosthesis related | N/A | Mean 3.2 (range, 2–5.1) | Domed tibia, mobile-bearing, lateral UKA | |
| Oxford Knee Score | 42 (range, 23–48) | N/A | Mean 2.3 (range, 0.7–4) | |||||||
| Xing et al. [40] | 2012 | 140 | 31 | 147 | Survivorship | None revised | 6 converted to TKA | Lateral UKA showed a trend towards less complications and implant failure | Mean 2.8 (range, 1.4–5.5) | |
| WOMAC scores | Similar to medial UKA | Similar to lateral UKA | NS | |||||||
| Heyse et al. [17] | 2012 | 223 | 50 | 173 | Survivorship | 3 revisions (of 50) (6%) | 12 revisions (of 173) (6.9%) | NS | Genesis UKA, now known as Accuris | |
| Knee Society knee and function scores | Reported for several subgroups, but not for the whole group | Reported for several subgroups, but not for the whole group | NS (not reported by the authors, but calculated from the data reported) | Mean 6.4 | ||||||
| Lustig et al. [24] | 2012 | 13 | 13 | 0 | Survivorship | 100% at 10 years; 80% at 15 years | N/A | Mean 10.2 (range, 3–22.1) | Three different implants | |
| Knee Society knee score | 88 (range, 65–100) | |||||||||
| Knee Society function score | 87 (range, 35–100) | |||||||||
| Lustig et al. [23] | 2011 | 52 | 54 | 0 | Survivorship | 98.08% at 10 years | NA | Mean 8.4 (range, 5–16) | HLS evoluktion uni, fixed all-polyethylene bearing | |
| Knee Society knee score | 95(range, 70–100) | |||||||||
| Knee Society function score | 82 (range, 25–100) | |||||||||
| Argenson et al. [5] | 2008 | 39 | 40 | 0 | Survivorship | 92% at 10 years, 84% at 16 years | N/A | Mean 12.6 (range, 3–23) | Cemented metal-backed | |
| Sah and Scott [34] | 2007 | 45 | 49 | 0 | Survivorship | 100% | N/A | Mean 5.2 | Brigham unicondylar, PFC, Preservation | |
| Knee Society knee score | 89 | |||||||||
| Knee Society function score | 90 | |||||||||
| Pennington et al. [32] | 2006 | 29 | 0 | Survivorship | 100% | N/A | Mean 12.4 | Two different designs | ||
| Hospital for Special Surgery Scores | 93 (range, 82–100) | |||||||||
| O’Rourke et al. [30] | 2005 | 103 | 14 | 122 | Survivorship | Not stated seperately for medial and lateral | Not stated seperately for medial and lateral | Mean 24 | Marmor | |
| Hospital for Special Surgery Scores | Not stated seperately for medial and lateral | Not stated seperately for medial and lateral | ||||||||
| Ashraf et al. [6] | 2002 | 79 | 83 | 0 | Survivorship | 83% at 10 years, 74% at 15 years | N/A | Mean 9 | St Georg Sled | |
| WOMAC | 78 (SD 9.1) | |||||||||
| Ohdera et al. [31] | 2001 | 17 | 18 | 0 | Survivorship | Cannot be calculated by data from the article | N/A | Mean 8.9 | Marmor, Oxford, PCA, Omnifit | |
| Japanese Orthopaedic Associataion knee score | 85.3 (SD 9.9) | |||||||||
| Gunther et al. [16] | 1996 | 51 | 53 | 0 | Survivorship | 82% at 5 years | N/A | Mean 5.2 | Oxford | |
| Marmor [27] | 1984 | 14 | 14 | 0 | Survivorship | 93% at 7.4 years | N/A | Mean 7.4 | All-polyethylene tibia | |
| Scott and Santore [36] | 1981 | 100 | 12 | 88 | Survivorship | 83% at 3.5 years | 99% at 3.5 years | Mean 3.5 (range, 2–6) | All-polyethylene tibia | |
| Current study | 2013 | 518 | 117 | 401 | Survivorship | 82.8% (range, 74.2%–93.4%) at 9 years | 89.5% (86–93%) at 9 years | NS | Mean 6 (range, 2.1–9.8) | Preservation cemented mobile bearing |
| WOMAC physical function | 33.6 (SD 29.5) | 23.4 (SD 24.3) | p = 0.030 | |||||||
| WOMAC pain | 33.7 (SD 31.3) | 21.3 (SD 26.5) | p = 0.003 | |||||||
| WOMAC stiffness | 22.8 (SD27.1) | 21.9 (SD 27.1) | p = 0.962 |
UKA = unicompartmental knee arthroplasty; N/A = not available; NS = nonsignificant.
We found no difference in survival between the three phases of surgical technique. However, when looking at the survival plot, one could get the impression that earlier surgical phases are associated with earlier failure and that the lack of statistical difference is likely attributable to the low number of revisions. Because the changes in surgical technique were implemented early on, this analysis does not allow assessment of whether the improved results achieved during the course of the study were achieved by the changes in surgical technique from Phase I to Phase II or whether they reflect the effect of a learning curve.
The percentage of lateral UKAs was 23% in our study, which is more than double the rate of 10% reported in the literature [18]. The reasons for this are speculative. Because a lateral UKA is considered a technically demanding and less frequent procedure, we assume in our cohort a lateral UKA might have been performed in patients when other surgeons might have used a TKA instead.
Our study, which is the largest comparing medial with lateral UKAs, suggests a medial UKA is associated with superior HRQoL when compared with a lateral UKA. Nevertheless, a lateral UKA provides good physical function and pain relief and is associated with similar implant survival as a medial UKA. Further research is needed to evaluate if the differences in HRQoL are attributable to the procedure (lateral UKA) or to the disease (lateral osteoarthritis). Although this is also the largest study analyzing phases in surgical technique in UKAs, the number of revisions is still too small to detect any association with implant survival. The survival rate of the implant used in this study was 88% at 9 years, which compares favorably to rates reported in the literature and to available registry data.
Footnotes
One of the authors (TRL) certifies that he has or may receive payments or benefits, during the study period, an amount of USD 10,000–USD 100,000, from DePuy International Ltd (Leeds, UK).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Department of Surgery, Wedel Hospital, Wedel, Germany, and Department of Orthopaedic Surgery, Asklepios Westklinikum Hamburg, Hamburg, Germany.
References
- 1.Altman DG, Bland JM. Statistics notes. Units of analysis. BMJ. 1997;314:1874. doi: 10.1136/bmj.314.7098.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, Gotzsche PC, Lang T. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 3.Altuntas AO, Alsop H, Cobb JP. Early results of a domed tibia, mobile bearing lateral unicompartmental knee arthroplasty from an independent centre. Knee. 2012 Dec 28. pii: S0968-0160(12)00230-X. doi: 10.1016/j.knee.2012.11.008 [Epub ahead of print]. [DOI] [PubMed]
- 4.Arastu MH, Vijayaraghavan J, Chissel H, Hull JB, Newmann JH, Robinson JR. Early failure of a mobile-bearing unicompartmental knee replacement. Knee Surg Sports Traumatol Arthrosc. 2009;17:1178–1183. doi: 10.1007/s00167-009-0779-z. [DOI] [PubMed] [Google Scholar]
- 5.Argenson JN, Parratte S, Bertani A, Flecher X, Aubaniac JM. Long-term results with a lateral unicondylar replacement. Clin Orthop Relat Res. 2008;466:2686–2693. doi: 10.1007/s11999-008-0351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashraf T, Newman JH, Evans RL, Ackroyd CE. Lateral unicompartmental knee replacement survivorship and clinical experience over 21 years. J Bone Joint Surg Br. 2002;84:1126–1130. doi: 10.1302/0301-620X.84B8.13447. [DOI] [PubMed] [Google Scholar]
- 7.Australian Orthopaedic Association National Joint Replacement Registry. Annual Report. 2009. Adelaide, Australia: Australian Orthopaedic Association. Available at: https://aoanjrr.dmac.adelaide.edu.au/de/annual-reports-2009. Accessed March 13, 2013.
- 8.Australian Orthopaedic Association National Joint Replacement Registry. Annual Report. 2012. Adelaide, Australia: Australian Orthopaedic Association. Available at: https://aoanjrr.dmac.adelaide.edu.au/de/annual-reports-2012. Accessed March 13, 2013.
- 9.Barret M, Wilson E, Whalen D. Summary 2007 HCUP Nationwide Inpatient Sample (NIS) Comparison Report. Report # 2010-03. Washington, DC, USA: Agency for Healthcare Research and Quality; September 9, 2010. HCUP Method Series Report.
- 10.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 11.Bullinger M. German translation and psychometric testing of the SF-36 Health Survey: preliminary results from the IQOLA Project. International Quality of Life Assessment. Soc Sci Med. 1995;41:1359–1366. doi: 10.1016/0277-9536(95)00115-N. [DOI] [PubMed] [Google Scholar]
- 12.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ, USA: Lawrence Erlbaum Associates Inc; 1988. [Google Scholar]
- 13.Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86:963–974. doi: 10.2106/00004623-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Fitz W, Scott RD. Unicompartmental knee arthroplasty. In: Scott WN, editor. Insall & Scott Surgery of the Knee. Philadelphia, PA, USA: Elsevier Churchill Livingstone; 2012. pp. 988–995. [Google Scholar]
- 15.Geller JA, Yoon RS, McKean J, Macaulay W. Does a high-flexion design affect early outcome of medial unicondylar knee arthroplasty? Clinical comparison at 2 years. J Arthroplasty. 2011;26:1468–1474. doi: 10.1016/j.arth.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 16.Gunther TV, Murray DW, Miller R, Wallace AJ, Carr AJ, O’Connor JJ, McLardy-Smith P, Goodfellow J. Lateral unicompartmental arthroplasty with the Oxford meniscal knee. Knee. 1996;3:33–39. doi: 10.1016/0968-0160(96)00208-6. [DOI] [Google Scholar]
- 17.Heyse TJ, Khefacha A, Peersman G, Cartier P. Survivorship of UKA in the middle-aged. Knee. 2012;19:585–591. doi: 10.1016/j.knee.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Heyse TJ, Tibesku CO. Lateral unicompartmental knee arthroplasty: a review. Arch Orthop Trauma Surg. 2010;130:1539–1548. doi: 10.1007/s00402-010-1137-9. [DOI] [PubMed] [Google Scholar]
- 19.König DP, Popken F, Herzberg W, Eysel P. The minimally invasive unicompartimental knee system ‘Preservation’. First clinical results and analysis of complications. Orthopade. 2004;33:1284–1289. doi: 10.1007/s00132-004-0716-2. [DOI] [PubMed] [Google Scholar]
- 20.Lequesne MG, Mery C, Samson M, Gerard P. Indexes of severity for osteoarthritis of the hip and knee. Validation—value in comparison with other assessment tests. Scand J Rheumatol Suppl. 1987;65:85–89. doi: 10.3109/03009748709102182. [DOI] [PubMed] [Google Scholar]
- 21.Lisowski LA, van den Bekerom MP, Pilot P, van Dijk CN, Lisowski AE. Oxford Phase 3 unicompartmental knee arthroplasty: medium-term results of a minimally invasive surgical procedure. Knee Surg Sports Traumatol Arthrosc. 2011;19:277–284. doi: 10.1007/s00167-010-1213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludwig FJ, Melzer C, Grimmig H, Daalmann HH. [Cross cultural adaptation of the lequesne algofunctional indices for German speaking patients with osteoarthritis of the hip and the knee] [in German] Rehabilitation (Stuttg). 2002;41:249–257. doi: 10.1055/s-2002-33273. [DOI] [PubMed] [Google Scholar]
- 23.Lustig S, Elguindy A, Servien E, Fary C, Munini E, Demey G, Neyret P. 5- to 16-year follow-up of 54 consecutive lateral unicondylar knee arthroplasties with a fixed-all polyethylene bearing. J Arthroplasty. 2011;26:1318–1325. doi: 10.1016/j.arth.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Lustig S, Parratte S, Magnussen RA, Argenson JN, Neyret P. Lateral unicompartmental knee arthroplasty relieves pain and improves function in posttraumatic osteoarthritis. Clin Orthop Relat Res. 2012;470:69–76. doi: 10.1007/s11999-011-1963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons MC, Macdonald SJ, Somerville LE, Naudie DD, McCalden RW. Unicompartmental versus total knee arthroplasty database analysis: is there a winner? Clin Orthop Relat Res. 2012;470:84–90. doi: 10.1007/s11999-011-2144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mariani EM, Bourne MH, Jackson RT, Jackson ST, Jones P. Early failure of unicompartmental knee arthroplasty. J Arthroplasty. 2007;22(Suppl 2):81–84. doi: 10.1016/j.arth.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Marmor L. Lateral compartment arthroplasty of the knee. Clin Orthop Relat Res. 1984;186:115–121. [PubMed] [Google Scholar]
- 28.9th Annual Report 2012. Hernel Hempstead, Hertfordshire, UK: NJR Centre; 2012. [Google Scholar]
- 29.NIH Consensus Panel NIH Consensus Statement on Total Knee Replacement Dec 8–10, 2003. J Bone Joint Surg Am. 2004;86:1328–1335. doi: 10.2106/00004623-200406000-00031. [DOI] [PubMed] [Google Scholar]
- 30.O’Rourke MR, Gardner JJ, Callaghan JJ, Liu SS, Goetz DD, Vittetoe DA, Sullivan PM, Johnston RC. The John Insall Award: unicompartmental knee replacement: a minimum twenty-one-year followup, end-result study. Clin Orthop Relat Res. 2005;440:27–37. doi: 10.1097/01.blo.0000185451.96987.aa. [DOI] [PubMed] [Google Scholar]
- 31.Ohdera T, Tokunaga J, Kobayashi A. Unicompartmental knee arthroplasty for lateral gonarthrosis: midterm results. J Arthroplasty. 2001;16:196–200. doi: 10.1054/arth.2001.2090. [DOI] [PubMed] [Google Scholar]
- 32.Pennington DW, Swienckowski JJ, Lutes WB, Drake GN. Lateral unicompartmental knee arthroplasty: survivorship and technical considerations at an average follow-up of 12.4 years. J Arthroplasty. 2006;21:13–17. doi: 10.1016/j.arth.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Sackett DL, Richardson WS, Rosenberg W. Evidence-based Medicine: How to Practice and Teach EBM. New York, NY, USA: Churchill Livingstone; 1997. [Google Scholar]
- 34.Sah AP, Scott RD. Lateral unicompartmental knee arthroplasty through a medial approach. Study with an average five-year follow-up. J Bone Joint Surg Am. 2007;89:1948–1954. doi: 10.2106/JBJS.F.01457. [DOI] [PubMed] [Google Scholar]
- 35.Scott RD. Lateral unicompartmental replacement: a road less traveled. Orthopedics. 2005;28:983–984. doi: 10.3928/0147-7447-20050901-34. [DOI] [PubMed] [Google Scholar]
- 36.Scott RD, Santore RF. Unicondylar unicompartmental replacement for osteoarthritis of the knee. J Bone Joint Surg Am. 1981;63:536–544. [PubMed] [Google Scholar]
- 37.Stucki G, Meier D, Stucki S, Michel BA, Tyndall AG, Dick W, Theiler R. [Evaluation of a German version of WOMAC (Western Ontario and McMaster Universities) Arthrosis Index] [in German] Z Rheumatol. 1996;55:40–49. [PubMed] [Google Scholar]
- 38.Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(Suppl):AS264–AS279. [PubMed] [Google Scholar]
- 39.Whiteside LA. Making your next unicompartmental knee arthroplasty last: three keys to success. J Arthroplasty. 2005;20(Suppl 2):2–3. doi: 10.1016/j.arth.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 40.Xing Z, Katz J, Jiranek W. Unicompartmental knee arthroplasty: factors influencing the outcome. J Knee Surg. 2012;25:369–374. doi: 10.1055/s-0031-1299666. [DOI] [PubMed] [Google Scholar]