Abstract
Data are equivocal on whether voluntary activation is preserved or decreased in old compared to young adults. Further, data are scant on the effect of age on the rate of muscle relaxation when the muscle is contracting voluntarily. Assessment of both measures with transcranial magnetic stimulation (TMS) yields information which cannot be obtained with traditional peripheral nerve stimulation. Hence, voluntary activation and peak relaxation rate of the elbow flexors were assessed with TMS during repeated maximal efforts in 30 men and 28 women between the ages of 22–84 years. Voluntary activation was similar for the two sexes (P = 0.154) and was not affected by age in men (96.2 ± 2.7 %; P = 0.887) or women (95.1 ± 3.0 %; P = 0.546). Men had a significantly faster peak rate of relaxation than women in absolute units (−880.0 ± 223.2 vs. −360.2 ± 78.5 Nm/ s, respectively; P < 0.001) and when normalized to subject strength (−12.5 ± 2.1 vs. −8.7 ± 1.0 s−1, respectively; P < 0.001). Absolute and normalized relaxation rates slowed with age in men (P = 0.002 and P = 0.006, respectively), but not women (P = 0.142 and P = 0.950, respectively). Across the age range studied, all subjects, regardless of age or sex, were able to achieve high voluntary activation scores for the elbow flexors (~95 %). In contrast, peak relaxation rate was markedly faster in men than women and slowed with age in men but not women. Normalization of relaxation rates to strength did not affect the influence of age or sex.
Keywords: Isometric strength, Transcranial magnetic stimulation, Twitch interpolation
Introduction
Transcranial magnetic stimulation (TMS) is a non-invasive technique that can be used to excite or inhibit different cortical areas of the human brain. When TMS of sufficient intensity is applied to the motor cortex during a voluntary contraction, it induces a transient excitation followed by a longer-lasting inhibition (~50–250 ms) of cortical neurons (e.g., Barker et al. 1985; Day et al. 1989; Fuhr et al. 1991). The excitation and inhibition result in both electrical (EMG) and mechanical (torque or force) responses which can be recorded at the target muscle or joint. The EMG response to excitation is termed the motor evoked potential (MEP) whereas the torque response is termed the superimposed twitch. After the MEP, there is an inhibition of ongoing voluntary EMG which is termed the silent period. As a result of this withdrawal of voluntary drive, muscle fibers which were contracting volitionally are made to relax and torque decreases. There are thousands of studies which have used TMS but the overwhelming majority have ignored the changes to torque output. However, the superimposed twitch can be used to quantify voluntary activation (e.g., Todd et al. 2003, 2004) and the rate of muscle relaxation allows in vivo assessment of how a major muscle property changes with fatigue, age, temperature, disease, etc. (Hunter et al. 2006, 2008; Todd et al. 2005, 2007). The superimposed twitch and relaxation rate evoked by TMS yield useful information which cannot be obtained from an electrical stimulus to a peripheral motor nerve.
Since Merton developed the interpolated twitch technique in 1954 (Merton 1954), there has been considerable interest and investigation into the capacity of humans to activate their muscles during a maximal voluntary contraction (MVC). This question is typically addressed by the application of an electrical stimulus to the peripheral nerve which supplies the muscle/muscle group of interest (see Gandevia 2001 for review). During an MVC, if the stimulus evokes a superimposed twitch it is evident that the subject was not able to recruit all motor units voluntarily or that the motor unit firing rates were insufficient to produce optimal fusion of torque (e.g., Belanger and McComas 1981; Herbert and Gandevia 1999). However, as the presence of a superimposed twitch identifies a failure within the nervous system at or above the level of stimulation, a peripheral nerve stimulus provides little insight into the site of failure. Hence, one advantage of TMS over peripheral nerve stimulation for the assessment of voluntary activation is that a superimposed twitch is indicative of a suboptimal output from the motor cortex (Gandevia et al. 1996; Taylor et al. 2000; Taylor and Gandevia 2001; Todd et al. 2003, 2004, 2005). This technique was first developed and validated for the elbow flexors (Todd et al. 2003, 2004) but has since been applied to a variety of muscle groups (Lee et al. 2008; Sidhu et al. 2009; van Duinen et al. 2010).
The rate at which a muscle relaxes has an important role in how the nervous and muscular systems interact. A muscle which relaxes slowly requires the input of fewer impulses per second from motoneurons to achieve a given muscle torque than a muscle which relaxes quickly (Henneman 1980). Muscle relaxation has classically been measured from the torque response to an electrical stimulus delivered to a peripheral nerve when the muscle is relaxed. Although such data are useful, they provide no information about the properties of a muscle in its most functionally relevant state; i.e., during a voluntary contraction. We have recently addressed this limitation by using TMS during a voluntary contraction to assess muscle relaxation in vivo (Todd et al. 2005, 2007). Beyond the applications to fatigue or aging, this technique also has clinical utility (Kleine and Stegeman 2007) for conditions such as myotonic dystrophy, Brody’s disease and malignant hyperthermia.
Healthy aging causes a multitude of changes to the neuromuscular system (see (Doherty 2003 for review) but data from the elbow flexors are equivocal. For example, there are a nearly equivalent number of studies which report that voluntary activation is either unaffected by age (e.g., Allman and Rice 2001; Dalton et al. 2010; Hunter et al. 2008; Jakobi and Rice 2002; Klein et al. 2001) or reduced in the old compared to young (e.g., Bilodeau et al. 2001; De Serres and Enoka 1998; Yoon et al. 2008; Yue et al. 1999). Further, muscle relaxation of the elbow flexors is unaffected by age when assessed using peripheral nerve stimulation at rest (Allman and Rice 2001; Dalton et al. 2010; Doherty et al. 1993) but is markedly slower in old than young adults when tested by TMS during an MVC (Hunter et al. 2008). Each of these studies was limited to a comparison between two groups of subjects and only one study assessed voluntary activation and relaxation with TMS (Hunter et al. 2008). Thus, our aim was to use TMS to collect elbow flexor voluntary activation and muscle relaxation data from men and women across a spectrum of the adult lifespan. We hypothesized that voluntary activation would be unaffected by age in both sexes but that the rate of muscle relaxation would slow progressively with age.
Methods
Test subjects
Fifty-eight subjects (30 men and 28 women) volunteered for this study. All were healthy with neither contraindications for TMS nor a musculoskeletal impairment of their dominant arm. Height and body mass were measured and handedness was assessed using the Edinburgh inventory (Oldfield 1971). Physical activity was quantified using the General Practice Physical Activity Questionnaire (Department of Health 2009). Women were not asked about menstrual status or history of hormone replacement therapy. Mean data for age, height, body mass, body mass index, and physical activity for men and women in each decade of life studied are provided in Table 1. All subjects signed a consent form prior to participation. Experimental procedures were approved by the institutional ethics committee and conducted according to the Declaration of Helsinki at Neuroscience Research Australia.
Table 1.
Subject characteristics categorized by decade and sex
| Decade (n = M, F) | 3rd (n = 5, 5) | 4th (n = 5, 5) | 5th (n = 5, 4) | 6th (n = 5, 4) | 7th (n = 6, 6) | 8th (n = 4, 2) | 9th (n = 0, 2) | |
|---|---|---|---|---|---|---|---|---|
| Characteristic | Sex | |||||||
| Age (years) | M | 25.8 ± 2.7 | 33.6 ± 1.9 | 44.0 ± 2.7 | 55.3 ± 2.3 | 63.6 ± 2.1 | 73.9 ± 2.7 | – |
| F | 26.5 ± 2.1 | 33.9 ± 3.9 | 43.4 ± 2.9 | 51.9 ± 1.8 | 64.1 ± 3.7 | 74.4 ± 0.9 | 82.7 ± 1.9 | |
| Height (cm) | M | 176.1 ± 4.1 | 177.7 ± 7.6 | 172.6 ± 7.0 | 175.8 ± 5.1 | 177.0 ± 4.9 | 167.6 ± 6.6 | – |
| F | 164.8 ± 4.7 | 168.4 ± 12.7 | 164.4 ± 2.1 | 167.3 ± 4.9 | 160.3 ± 4.4 | 153.4 ± 7.2 | 161.0 ± 1.4 | |
| Body mass (kg) | M | 70.2 ± 5.8 | 74.6 ± 7.0 | 77.6 ± 8.3 | 73.0 ± 10.7 | 75.0 ± 7.8 | 80.5 ± 4.4 | – |
| F | 59.1 ± 7.4 | 67.0 ± 13.7 | 65.3 ± 4.3 | 69.0 ± 10.7 | 66.3 ± 14.2 | 69.5 ± 13.4 | 67.0 ± 9.9 | |
| BMI (kg/m2) | M | 22.6 ± 2.2 | 23.6 ± 0.8 | 26.3 ± 4.7 | 23.6 ± 3.6 | 23.9 ± 2.2 | 28.8 ± 3.2 | – |
| F | 21.8 ± 2.9 | 23.5 ± 3.5 | 24.2 ± 1.6 | 24.6 ± 2.8 | 25.9 ± 5.7 | 29.9 ± 8.5 | 25.8 ± 3.4 | |
| PAI (1–4) | M | 3.4 ± 0.5 | 3.4 ± 1.3 | 3.4 ± 0.9 | 3.8 ± 0.4 | 3.5 ± 1.2 | 3.8 ± 0.5 | – |
| F | 3.2 ± 1.3 | 2.6 ± 1.1 | 3.5 ± 0.6 | 3.8 ± 0.5 | 4.0 ± 0.0 | 3.5 ± 0.7 | 3.0 ± 0.0 | |
Values are means ± SD
n number of subjects, BMI body mass index, PAI physical activity index (1 = inactive, 2 = moderately inactive, 3 = moderately active, and 4 = active)
Recordings
Participants were seated with their dominant arm in an isometric myograph and an angle of 90° at both the elbow and shoulder. The forearm was supinated and securely strapped just proximal to the wrist. The torque produced by elbow flexion was measured with a calibrated linear strain gauge (Xtran, Melbourne, Australia). Adhesive surface electrodes (Ag–AgCl, 10 mm diameter) were used to record EMG from the biceps brachii and triceps brachii muscles. Skin was thoroughly cleaned using alcohol swabs before placement of the electrodes. Electrodes were arranged in a monopolar configuration with the recording electrode over the middle of the muscle belly and the reference electrode over the distal tendon. Torque and EMG signals were sampled at 1,000 and 2,000 Hz, respectively. EMG signals were amplified (×100) and bandpass filtered (16–1,000 Hz) using CED 1902 amplifiers (Cambridge Electronic Design Ltd., Cambridge, UK). The EMG and torque signals were recorded to computer using a 12-bit A/D converter (CED 1401 Plus; Cambridge Electronic Design) and Spike2 software (version 6.06; Cambridge Electronic Design).
Stimulation
Electrical stimulation
Using a constant current stimulator (Digitimer, model DS7AH, Welwyn Garden City, UK), the brachial plexus was electrically stimulated to assess the maximum compound muscle action potential (Mmax) of the biceps and triceps brachii muscles. Single stimuli (100-μs duration) were delivered via surface electrodes (Ag–AgCl, 10 mm diameter). The cathode was placed in the supraclavicular fossa (Erb’s point) and the anode on the acromion. In each subject, the initial stimulus intensity was 20 mA and was increased in steps of 20 mA until the amplitude of the biceps and triceps potentials reached a maximum (range of 80–420 mA).
TMS
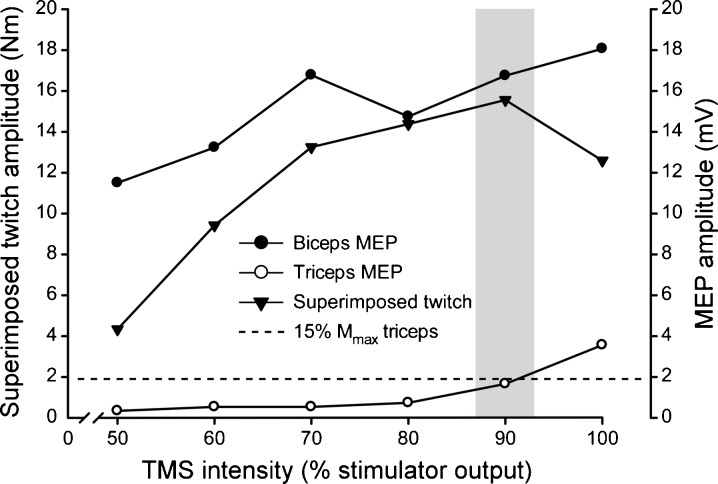
A circular coil (13.5 cm; Magstim 200, Magstim, Whitland, UK) was placed over the vertex to produce an MEP in the biceps and triceps. The direction of current flow in the coil preferentially activated the motor cortex in the hemisphere which innervated the dominant arm. The stimulus intensity during the assessment of voluntary activation was set to produce the largest superimposed twitch during brief efforts at 50 % of MVC, while keeping the amplitude of the triceps MEP below 15 % Mmax (range of 40–100 % of maximal output; Fig. 1). This intensity was chosen because an intensity which is too low (as indicated by the size of the superimposed twitch), would cause the extrapolated resting twitch and thus the voluntary activation of a subject to be underestimated. However, an intensity which is too high (as indicated by the size of the triceps MEP) would cause strong activation of the elbow extensors during an MVC. This antagonist activity would reduce the size of the elbow flexor superimposed twitch and lead to an overestimate of voluntary activation (Todd et al. 2004). To confirm that the chosen intensity was not set too high a stimulus was delivered during a brief (~2 s) MVC to measure the amplitude of the triceps MEP. If the amplitude exceeded 15 % Mmax, brief MVCs were performed with progressively lower stimulus intensities until the triceps MEP was acceptably small. To assess peak relaxation rate, the stimulus intensity (range of 60–100 % of maximal output) was set to the level which produced a silent period of approximately 200 ms during a brief MVC (mean of 202.7 ± 39.0 ms across all subjects).
Fig. 1.
Influence of stimulus intensity on amplitudes of the superimposed twitch and motor evoked potentials of the biceps and triceps. Data were recorded from a single subject (48-year old man) during multiple efforts at 50 % MVC to determine the optimal TMS intensity for the part of the protocol concerned with calculating voluntary activation. The optimal intensity (denoted by the shaded bar) was that which generated the greatest superimposed twitch amplitude whilst keeping the triceps MEP < 15 % the amplitude of the triceps compound muscle action potential (Mmax) during a contraction at 100 % MVC (dotted horizontal line)
Experimental protocol
Each subject completed the experiment during one session. Data collection began with the determination of Mmax in both biceps and triceps. Two or three brief elbow flexor MVCs were then performed to determine maximal torque. Visual feedback (via a series of light-emitting diodes) and verbal encouragement were given during all maximal efforts. Submaximal targets of 50 and 75 % MVC were calculated based on the peak torque attained and these targets were displayed on the visual feedback device. The remainder of the protocol was divided into two parts, measurement of voluntary activation and measurement of the peak rate of muscle relaxation.
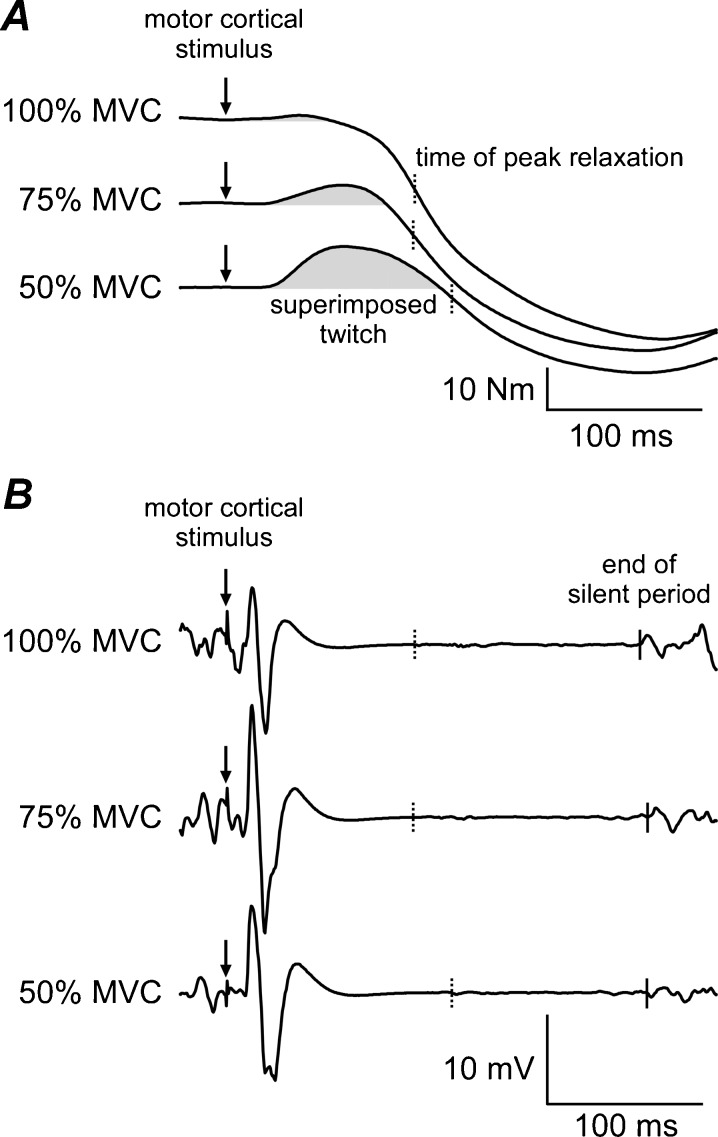
In the voluntary activation part of the protocol, subjects performed five sets of three brief (~2 s) contractions. Two minutes of rest were given between sets and 6 s of rest between contractions within a set. Each set involved an MVC and a submaximal effort at 50 and 75 % MVC. The MVC was always performed first but the order of submaximal efforts alternated between sets. TMS was delivered manually by an investigator when the subject reached peak torque during the MVCs or was stable at the target torque during the submaximal efforts. The silent period which followed the stimulus caused a transient drop in torque and subjects were asked to quickly return to the pre-stimulus torque before relaxing. Raw traces of torque and EMG responses to TMS at the three different contraction strengths are displayed for a single subject in Fig. 2.
Fig. 2.
Raw traces of elbow flexor torque and EMG responses to stimulation of the motor cortex during voluntary contractions at 50, 75 and 100 % maximal torque. Arrows indicate timing of the motor cortical stimulus. A, torque traces from a single subject (56-year-old man) showing the superimposed twitch (shaded area) and subsequent loss of torque caused by muscle relaxation. Dotted vertical lines indicate the time at which the peak relaxation rate occurred. B, corresponding biceps EMG traces showing motor-evoked potentials and silent periods. Solid vertical lines indicate the end of the silent period
In the muscle relaxation part of the protocol, subjects performed five brief MVCs separated by 2 min of rest. TMS was delivered as above and subjects were reminded to contract maximally immediately after the stimulus before relaxing. Prior to starting this part of the protocol, silent period durations were determined from the MVCs performed in the previous part. If the silent period duration was close to 200 ms, the same intensity of stimulation was used in this part of the protocol. If not, brief MVCs were performed with stronger or weaker stimulus intensities until the silent period duration was ~200 ms.
Data analysis
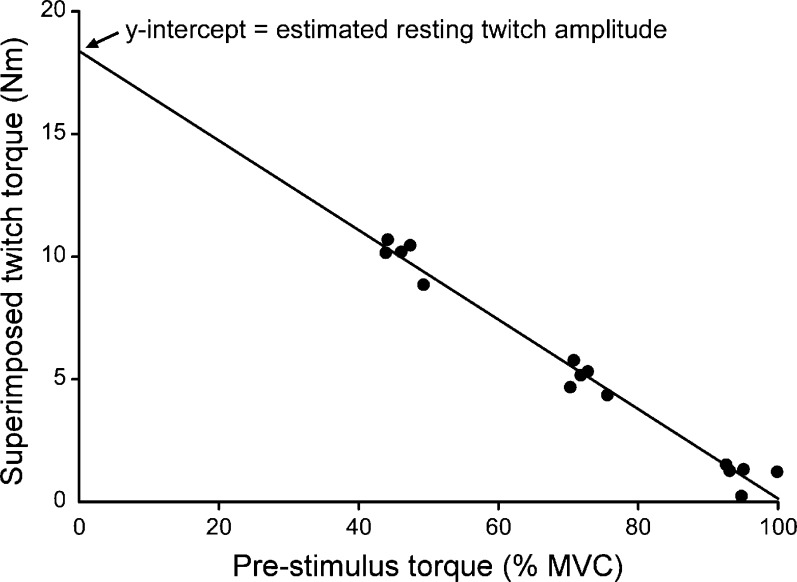
Responses to the physical activity questionnaire were converted to a numerical score in which 1 = inactive, 2 = moderately inactive, 3 = moderately active, and 4 = active. All EMG and torque measures were determined off-line using Signal software (version 4.06, Cambridge Electronic Design). Voluntary activation was calculated as previously described for TMS (Todd et al. 2003). In brief, in each subject, amplitudes of all 15 superimposed twitches were plotted against the torque (50, 75 or 100 % MVC) at which they were evoked (Fig. 3). A linear regression was applied to the data and the y-intercept was taken as the amplitude of the estimated resting twitch (Fig. 3, see also Todd et al. 2003). This value was then inserted into the following formula: 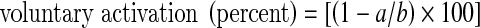 in which a represents the mean amplitude of the superimposed twitches at 100 % MVC and b represents the amplitude of the estimated resting twitch. A valid estimated resting twitch could not be obtained from one man (41 years of age) and two women (28 and 75 years of age) and so these subjects were excluded from the analyses of estimated resting twitch size and voluntary activation. Duration of the silent period was measured from the time of cortical stimulation until the return of voluntary EMG. Muscle relaxation was determined from the fall in elbow flexor torque during the silent period. The peak rate of relaxation was calculated as the negative slope over a 10-ms interval (5 ms either side of the steepest instantaneous slope; Hunter et al. 2008; Todd et al. 2005). To account for differences in strength between subjects, the absolute rate of relaxation was divided by the peak torque which preceded the relaxation (MVC plus any superimposed twitch). Mean values of the absolute and normalized rates of relaxation from the five MVCs are reported for each subject.
in which a represents the mean amplitude of the superimposed twitches at 100 % MVC and b represents the amplitude of the estimated resting twitch. A valid estimated resting twitch could not be obtained from one man (41 years of age) and two women (28 and 75 years of age) and so these subjects were excluded from the analyses of estimated resting twitch size and voluntary activation. Duration of the silent period was measured from the time of cortical stimulation until the return of voluntary EMG. Muscle relaxation was determined from the fall in elbow flexor torque during the silent period. The peak rate of relaxation was calculated as the negative slope over a 10-ms interval (5 ms either side of the steepest instantaneous slope; Hunter et al. 2008; Todd et al. 2005). To account for differences in strength between subjects, the absolute rate of relaxation was divided by the peak torque which preceded the relaxation (MVC plus any superimposed twitch). Mean values of the absolute and normalized rates of relaxation from the five MVCs are reported for each subject.
Fig. 3.
Calculation of the estimated resting twitch (ERT) in a single subject. A, data were recorded from a single subject (56-year-old man) during five sets of three contractions (one each at 50, 75 and 100 % MVC). Amplitude of the estimated resting twitch (ERT) is the y-intercept (18.4 Nm) of the extrapolated linear regression (r2 = 0.98)
Statistical analysis
For all variables except voluntary activation, an independent-samples t test was used (PASW version 18; SPSS Inc., Chicago, IL) to compare men and women irrespective of age. Linear regressions were then used to assess the effect of age on each variable separately for men and women. The effects of sex and age on voluntary activation were analyzed separately using Spearman non-parametric bivariate correlations. All data in the text and tables are reported as the mean ± SD. Statistical significance was set at P < 0.05.
Results
Subject characteristics
The means ± SD for men and women of each decade are provided in Table 1. Men were taller and heavier (both P < 0.001) than women but the groups had equivalent body mass and physical activity indices (P = 0.949 and P = 0.540, respectively). Men were of similar height (P = 0.142) and body mass (P = 0.170) across the age range whereas women were shorter with increasing age (r = 0.441, P = 0.019) but of similar body mass (P = 0.429). With increasing age, body mass index tended to be higher in men (r = 0.352, P = 0.057) and was statistically higher in women (r = 0.396, P = 0.037). Physical activity index did not differ in men (P = 0.420) or women (P = 0.203). All but two subjects were right-handed and all were tested on their dominant arm.
Maximal torque and voluntary activation
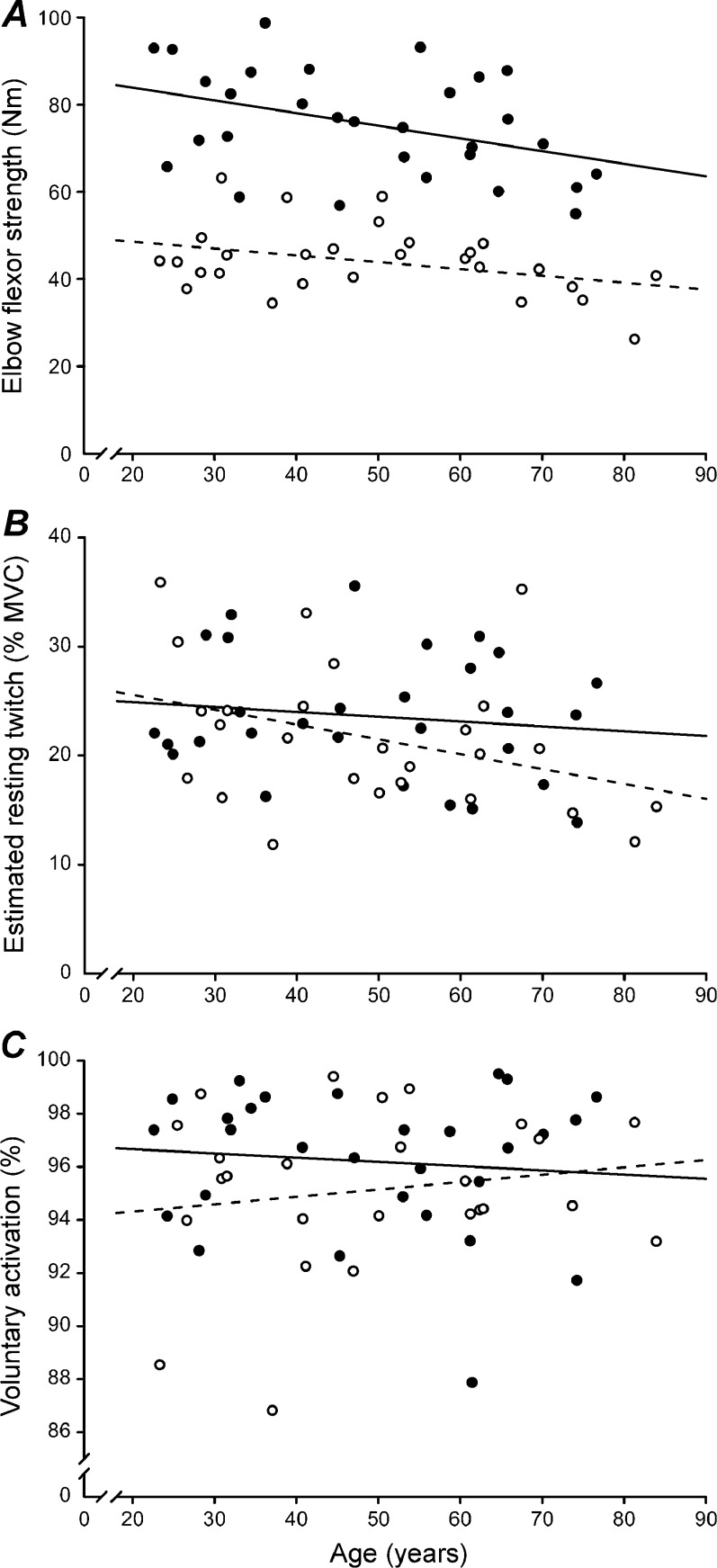
Men were stronger than women (75.4 ± 12.2 vs. 44.0 ± 7.9 Nm; P < 0.001). There was a significant age-related decrease of MVC torque in men (r = 0.399, P = 0.029) but the decrease only approached significance in women (r = 0.360, P = 0.060; Fig. 4a). The amplitude of the estimated resting twitch was significantly greater for men than women (16.8 ± 4.8 vs. 9.1 ± 3.0 Nm; P < 0.001) but not when expressed relative to MVC torque (23.6 ± 5.7 vs. 21.6 ± 6.6 %; P = 0.234). The size of the normalized estimated resting twitch was not different across the age range in men (P = 0.496) but showed a tendency to decrease in women (P = 0.066; Fig. 4b). Men and women achieved similar levels of voluntary activation (96.2 ± 2.7 vs. 95.1 ± 3.0 %, respectively; P = 0.154). Voluntary activation was similar across the age range in both men (P = 0.887) and women (P = 0.546; Fig. 4c).
Fig. 4.
Effect of age and sex on maximal torque, estimated resting twitch amplitude and voluntary activation. Data points represent peak values from individual men (n = 30 in A but 29 in B and C; filled circle) and women (n = 28 in A but 26 in B and C; empty circle) plotted against subject age. Linear regressions are displayed for men (solid line) and women (dashed line) separately. A, maximal torque was greater in men than women (P < 0.001). Maximal torque decreased with advancing age in men (P = 0.029) but only approached statistical significance in women (P = 0.060). B, expressed as a percentage of MVC torque, estimated resting twitch amplitude was similar in men and women (P = 0.234) and was unaffected by age in men (P = 0.496) and women (P = 0.066). C, voluntary activation was similar in men and women (P = 0.154) and unaffected by age in both men (P = 0.887) and women (P = 0.546)
Peak relaxation rate
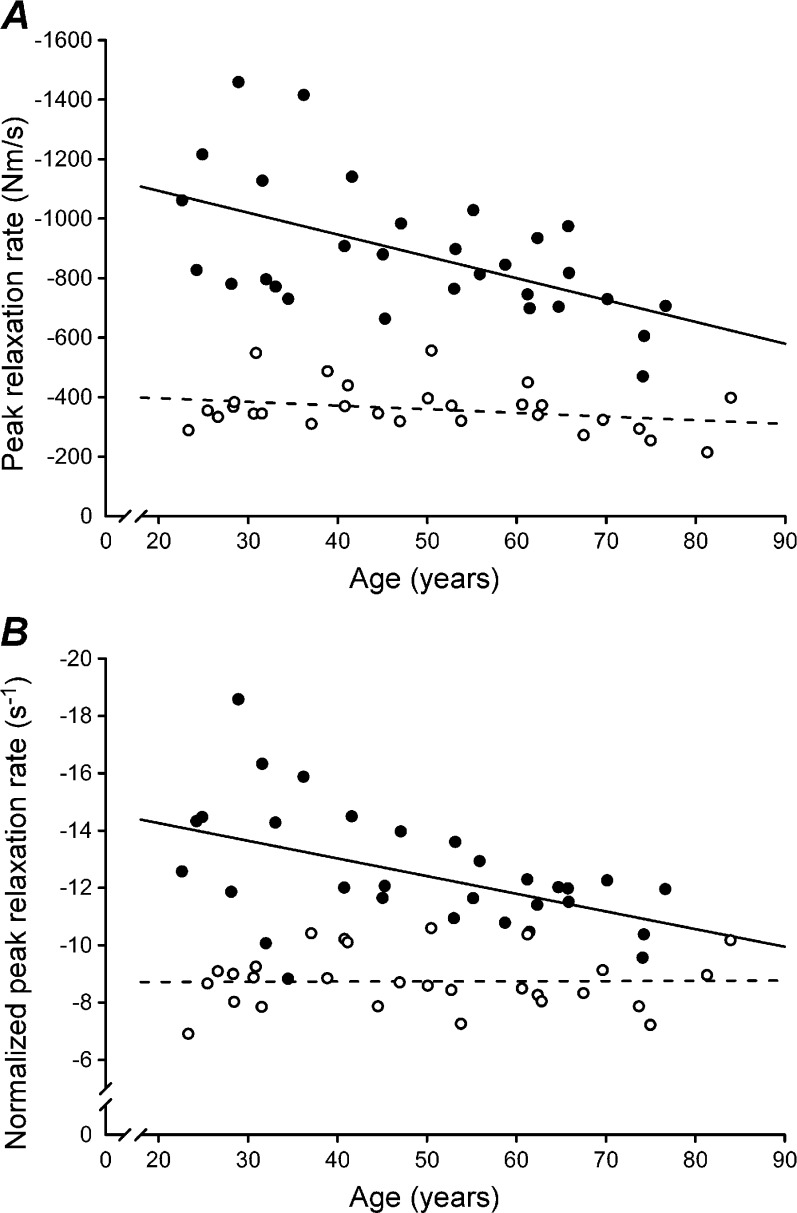
Absolute peak relaxation rate was significantly faster in men than women (−880.0 ± 223.2 vs. −360.2 ± 78.5 Nm/s; P < 0.001; Fig. 5a). The peak relaxation rate was significantly slower with increasing age in men (r = 0.549, P = 0.002) but not women (P = 0.142). After accounting for the greater strength in men, the normalized peak relaxation rate remained significantly faster in men than women (−12.5 ± 2.1 vs. −8.7 ± 1.0 s−1; P < 0.001). As with the absolute rate, normalized peak relaxation rate was slower with advancing age in men (r = 0.490, P = 0.006) but not women (P = 0.950; Fig. 5b).
Fig. 5.
Effect of age and sex on absolute and normalized peak relaxation rates. Data points represent peak values from individual men (n = 30; filled circle) and women (n = 28; empty circle) plotted against subject age. Linear regressions are displayed for men (solid line) and women (dashed line) separately. Note that the y-axes are inverted and that larger negative numbers indicate a faster muscle relaxation. A, The absolute peak relaxation rate was faster in men than women (P < 0.001). Peak relaxation rate slowed with advancing age in men (P = 0.002) but not women (P = 0.142). B, Normalized rates represent absolute rates from A divided by the peak torque prior to the relaxation. Similar to absolute data, the normalized peak relaxation rate was faster in men than women (P < 0.001) and slowed with age in men (P = 0.006) but not women (P = 0.950)
Other observations
Amplitude of biceps Mmax was larger in men than in women (19.1 ± 5.1 vs. 12.0 ± 3.0 mV; P < 0.001). This effect of sex was also seen on the amplitude of the triceps Mmax (12.3 ± 3.4 vs. 7.2 ± 3.0 mV; P < 0.001). The amplitudes of both the biceps and triceps Mmax were progressively lower with increasing age in men (r = 0.579, P = 0.001 and r = 0.592, P = 0.001, respectively) and women (r = 0.552, P = 0.002 and r = 0.584, P = 0.001, respectively; Table 2). The onset latency of the biceps MEP was significantly prolonged in men compared to women (10.9 ± 0.9 vs. 10.0 ± 0.9 ms; P < 0.001; Table 2) but not when normalized to height (P = 0.329). Onset latency increased significantly with age in both men (r = 0.402, P = 0.027) and women (r = 0.526, P = 0.004; Table 2) even when normalized to height for men (r = 0.526, P = 0.003) and women (r = 0.676, P < 0.001).
Table 2.
Results categorized by decade and sex
| Decade (n = M,F) | 3rd (n = 5, 5/4) | 4th (n = 5, 5) | 5th (n = 5/4, 4) | 6th (n = 5, 4) | 7th (n = 6, 6) | 8th (n = 4, 2/1) | 9th (n = 0, 2) | |
|---|---|---|---|---|---|---|---|---|
| Result | Sex | |||||||
| MVC (Nm) | M | 81.5 ± 12.4 | 79.8 ± 15.1 | 75.4 ± 11.5 | 76.2 ± 11.9 | 74.7 ± 10.8 | 62.5 ± 6.7 | – |
| F | 43.2 ± 4.3 | 48.4 ± 12.0 | 42.8 ± 3.9 | 51.3 ± 5.8 | 42.9 ± 4.7 | 36.5 ± 2.2 | 33.3 ± 10.3 | |
| ERT (% MVC) | M | 23.0 ± 4.5 | 25.1 ± 6.8 | 26.0 ± 6.4 | 22.0 ± 6.0 | 24.6 ± 6.0 | 20.3 ± 5.8 | – |
| F | 27.0 ± 7.8 | 19.2 ± 5.2 | 25.6 ± 6.4 | 18.4 ± 1.8 | 23.1 ± 6.6 | 14.6 | 13.6 ± 2.3 | |
| VA (%) | M | 95.5 ± 2.4 | 98.2 ± 0.7 | 96.1 ± 2.5 | 95.9 ± 1.4 | 95.3 ± 4.4 | 96.3 ± 3.1 | – |
| F | 94.7 ± 4.6 | 94.1 ± 4.1 | 94.4 ± 3.4 | 97.1 ± 2.2 | 95.5 ± 1.5 | 95.4 | 95.4 ± 3.2 | |
| Peak RR (Nm/s) | M | 1065.6 ± 280.8 | 965.1 ± 295.9 | 911.8 ± 173.4 | 866.6 ± 101.1 | 809.2 ± 118.7 | 624.6 ± 118.4 | – |
| F | 342.4 ± 36.6 | 403.9 ± 104.2 | 365.2 ± 51.7 | 407.8 ± 102.0 | 352.5 ± 59.5 | 270.9 ± 27.8 | 303.2 ± 129.6 | |
| Norm. peak RR (s−1) | M | 14.3 ± 2.6 | 13.0 ± 3.4 | 12.8 ± 1.3 | 12.0 ± 1.2 | 11.6 ± 0.7 | 11.0 ± 1.3 | – |
| F | 8.3 ± 0.9 | 9.0 ± 0.9 | 9.2 ± 1.1 | 8.7 ± 1.4 | 8.7 ± 0.9 | 7.5 ± 0.5 | 9.5 ± 0.9 | |
| Time to peak RR (ms) | M | 90.7 ± 7.5 | 97.3 ± 9.7 | 90.9 ± 5.7 | 97.2 ± 3.5 | 106.7 ± 9.3 | 98.6 ± 7.0 | – |
| F | 98.0 ± 7.8 | 98.9 ± 8.4 | 104.3 ± 11.4 | 99.5 ± 3.6 | 103.7 ± 9.6 | 105.1 ± 0.7 | 101.4 ± 8.5 | |
| Biceps Mmax (mV) | M | 20.7 ± 3.4 | 23.0 ± 5.0 | 22.0 ± 7.1 | 19.5 ± 2.9 | 15.5 ± 2.0 | 13.6 ± 3.1 | – |
| F | 14.0 ± 4.0 | 12.9 ± 2.5 | 12.5 ± 1.4 | 12.3 ± 4.2 | 11.0 ± 1.4 | 0.7 ± 0.2 | 7.1 ± 0.6 | |
| Triceps Mmax (mV) | M | 15.0 ± 3.5 | 14.5 ± 4.2 | 12.0 ± 2.5 | 12.5 ± 2.8 | 11.1 ± 2.2 | 8.3 ± 1.5 | – |
| F | 10.0 ± 4.3 | 7.8 ± 1.9 | 7.1 ± 0.9 | 8.2 ± 2.7 | 6.2 ± 1.7 | 2.2 ± 0.0 | 4.9 ± 0.8 | |
| Biceps MEP latency (ms) | M | 10.2 ± 0.5 | 10.5 ± 1.1 | 11.0 ± 0.4 | 11.4 ± 1.3 | 11.1 ± 0.8 | 11.3 ± 0.8 | – |
| F | 9.0 ± 0.5 | 9.6 ± 0.6 | 10.4 ± 0.7 | 10.2 ± 1.0 | 10.2 ± 0.9 | 11.2 ± 0.2 | 10.0 ± 0.1 | |
Values are means ± SD
n number of subjects, MVC maximal voluntary contraction torque, ERT estimated resting twitch torque, VA voluntary activation, Peak RR peak relaxation rate, Norm. peak RR peak relaxation rate normalized to the torque prior to relaxation, Time to peak RR time from cortical stimulus until moment of peak relaxation, Biceps/Triceps Mmax amplitude of maximal resting compound muscle action potential, Biceps MEP latency time from cortical stimulus until the onset of the motor evoked potential
Discussion
The use of TMS has allowed us to study the effect of age and sex on voluntary drive and intrinsic muscle properties during maximal voluntary contractions of the elbow flexors. The first of the main findings is that voluntary activation of the elbow flexors is unaffected by either age or sex. Second, the peak relaxation rate of the elbow flexors is markedly faster in men than women and is slower with advancing age in men but not women. This age-related slowing is not seen with a resting twitch (Allman and Rice 2001; Dalton et al. 2010; Doherty et al. 1993) and so emphasizes the importance of the activity status of muscle fibers and the value of using TMS to assess muscle properties under physiologically relevant conditions. Among the major secondary findings was an age-related decrease of maximal torque which achieved statistical significance in men but just failed to do so in women (P = 0.06). For both sexes, elbow flexor strength was reasonably well-maintained until the eighth decade at which point it began to decline somewhat rapidly (Table 2), which is similar to previous findings of other muscle groups (e.g., Kallman et al. 1990; McNeil et al. 2007; Vandervoort and McComas 1986).
Voluntary activation
Although many subjects performed single elbow flexion maximal contractions in which the TMS did not evoke a superimposed twitch (i.e., MVCs with a voluntary activation score of 100 %), the mean activation score across 5 MVCs was below 100 % for all subjects. The mean value of 95.6 ± 3.1 % across all subjects is comparable to previous studies which used TMS (Hunter et al. 2006, 2008; Todd et al. 2003, 2004, 2005) but slightly less than 97–98 % commonly reported with peripheral nerve stimulation (Allman and Rice 2001; Bilodeau et al. 2001; Dalton et al. 2010; De Serres and Enoka 1998; Jakobi and Rice 2002; Klein et al. 2001; Todd et al. 2003; Yue et al. 1999). In the same subjects, a comparison of the two techniques revealed a lower activation score using TMS compared to peripheral nerve stimulation but the difference was not statistically significant and the authors noted caveats to comparing the two techniques (Todd et al. 2003). Although scores tended to be higher in men, there was not a statistically significant effect of sex on voluntary activation (Fig. 4c; Table 2). This is consistent with previous studies employing either TMS (Hunter et al. 2006) or peripheral nerve stimulation (e.g., Bilodeau et al. 2001; Miller et al. 1993).
In both men and women, the capacity to voluntarily activate the elbow flexors was maintained across the age range studied (Fig. 4c; Table 2). As noted in the Introduction, the data are equivocal concerning an age-related impairment of voluntary activation of the elbow flexors. Hence, our findings support (Allman and Rice 2001; Dalton et al. 2010; Hunter et al. 2008; Jakobi and Rice 2002; Klein et al. 2001) and oppose (Bilodeau et al. 2001; De Serres and Enoka 1998; Yoon et al. 2008; Yue et al. 1999) a similar number of published reports. Only one of these studies (Hunter et al. 2008) used TMS to assess the effect of age on voluntary activation and thus it is perhaps the most suitable for comparison. In that study, voluntary activation across five MVCs was ~4 % lower in a small sample of seven old adults (mean age of 73 years) compared to 17 young adults (mean age of 25 years). However, as previously described with peripheral motor nerve stimulation (Jakobi and Rice 2002), this age-related difference was greatest during the initial MVC attempts and abolished by the final MVC. Thus, insufficient task familiarization for old adults may contribute to the discrepancy concerning an age-related impairment to voluntary activation. It should be noted that conflicting results on the effect of age on voluntary activation are not unique to the elbow flexors because the same situation exists in other muscle groups as well (see Klass et al. 2007 for review). Ultimately, our position is that if methodological care is taken to optimize subject performance (see Gandevia 2001 for a list of six specific criteria), the capacity to voluntarily activate the elbow flexors will not decrease with age in healthy men and women.
Peak relaxation rate
Absolute peak relaxation rate, measured during the TMS-induced silent period, varied widely across subjects as the minimum and maximum values were −211 and −1,455 Nm/s, respectively. Spread of the data was reduced considerably by normalization of peak relaxation rate to torque (voluntary plus evoked) prior to the silent period. Minimum and maximum normalized values were −6.9 and −18.5 s−1, respectively. Absolute relaxation rates have not been published previously but the normalized peak relaxation rates of the present study are comparable to previous results for the elbow flexors (Hunter et al. 2006, 2008; Todd et al. 2005, 2007).
Although biopsy samples were not collected in this study, there are a number of published sex- and age-related changes which could account for our results. These include a larger cross-sectional area of type II muscle fibers in biceps of men than women (Edstrom and Nystrom 1969; Jennekens et al. 1971; Mattiello-Sverzut et al. 2003; Miller et al. 1993; Nygaard et al. 1983) which may explain the 44 % faster normalized relaxation rate observed in the men of this study. Age-related atrophy of type II muscle fibers in men (Klein et al. 2003; Klitgaard et al. 1990a; Mattiello-Sverzut et al. 2003) but not women (Mattiello-Sverzut et al. 2003) could account for the slowing of relaxation rate in men but not women (Fig. 5). Further, there is evidence that elderly men have a smaller proportion of type IIb (now referred to as IIx) muscle fibers (Monemi et al. 1998) as well as increased (Monemi et al. 1999) and decreased (Klitgaard et al. 1990b) levels of the type I and type IIb myosin heavy-chain isoforms, respectively. Other mechanisms which likely contribute to the age-related slowing of relaxation rate in men include an age-related reduction in calcium sequestering by the sarcoplasmic reticulum (Hunter et al. 1999) or an altered dissociation of the actin-myosin crossbridge (Fitts et al. 1984). All of these mechanisms remain speculative and so collection of relaxation rate data and biopsy samples from the same subjects warrants investigation. Less invasive than a biopsy would be a correlation of relaxation rate data to indirect estimates of myosin heavy-chain composition via the radial twitch response (Simunic et al. 2011).
Although we observed a slower normalized relaxation rate in elderly men compared to young men, the rate in the eighth decade was faster (−11.0 ± 1.3 s−1, n = 4) than observed previously (−9.5 ± 2.5 s−1, n = 5; Hunter et al. 2008). The sample sizes are small and so this may represent normal variability; but, there is also a methodological issue which is likely to play a role. The mean TMS intensity to calculate peak relaxation rate for the four men in the current study was 98 ± 5 % of stimulator output which is much higher than the 57 ± 10 % used in the prior study. In the earlier study, relaxation rates were not of primary interest and so a single TMS intensity was used which was optimized for the assessment of voluntary activation of the elbow flexors. To minimize activation of the antagonist elbow extensors, this stimulus intensity is moderate in most subjects (e.g., 50–70 % stimulator output). A comparable approach was used in the first part of the current protocol to assess voluntary activation. However, because relaxation rate data were of equal importance, we reset the TMS intensity to a level which produced a substantial interruption to descending drive (i.e., a silent period of ~200 ms) in order to obtain the maximal relaxation rate of the elbow flexors. In many subjects, this involved an increase of the TMS intensity from the first to second part of the protocol and a consequent speeding of relaxation rate.
Conclusions
The effect of age on one’s ability to drive their muscles during a maximal voluntary contraction is disputed. Present data indicate that if subjects are familiarized to the task, output from the motor cortex during maximal elbow flexion contractions is maintained with age in both men and women. Absolute and normalized peak relaxation rates are considerably faster in men than women and slow with age in men but not women. This study provides novel data of in vivo peak relaxation rates for men and women across much of the adult lifespan. These data and the protocol described here could be used in diagnosis and monitoring of conditions (see “Introduction”) involving abnormal muscle relaxation.
Acknowledgments
The authors thank Dr. Janet Taylor for helpful comments during the preparation of this manuscript.
References
- Allman BL, Rice CL. Incomplete recovery of voluntary isometric force after fatigue is not affected by old age. Muscle Nerve. 2001;24:1156–1167. doi: 10.1002/mus.1127. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/S0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Belanger AY, McComas AJ. Extent of motor unit activation during effort. J Appl Physiol. 1981;51:1131–1135. doi: 10.1152/jappl.1981.51.5.1131. [DOI] [PubMed] [Google Scholar]
- Bilodeau M, Erb MD, Nichols JM, Joiner KL, Weeks JB. Fatigue of elbow flexor muscles in younger and older adults. Muscle Nerve. 2001;24:98–106. doi: 10.1002/1097-4598(200101)24:1<98::AID-MUS11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Jakobi JM, Allman BL, Rice CL. Differential age-related changes in motor unit properties between elbow flexors and extensors. Acta Physiol (Oxf) 2010;200:45–55. doi: 10.1111/j.1748-1716.2010.02100.x. [DOI] [PubMed] [Google Scholar]
- Day BL, Rothwell JC, Thompson PD, Maertens de Noordhout A, Nakashima K, Shannon K, Marsden CD. Delay in the execution of voluntary movement by electrical or magnetic brain stimulation in intact man. Evidence for the storage of motor programs in the brain. Brain. 1989;112:649–663. doi: 10.1093/brain/112.3.649. [DOI] [PubMed] [Google Scholar]
- De Serres SJ, Enoka RM. Older adults can maximally activate the biceps brachii muscle by voluntary command. J Appl Physiol. 1998;84:284–291. doi: 10.1063/1.368025. [DOI] [PubMed] [Google Scholar]
- The General Practice Physical Activity Questionnaire (GPPAQ) London: National Health Service; 2009. [Google Scholar]
- Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. J Appl Physiol. 1993;74:868–874. doi: 10.1063/1.354879. [DOI] [PubMed] [Google Scholar]
- Edstrom L, Nystrom B. Histochemical types and sizes of fibres in normal human muscles. A biopsy study. Acta Neurol Scand. 1969;45:257–269. doi: 10.1111/j.1600-0404.1969.tb01238.x. [DOI] [PubMed] [Google Scholar]
- Fitts RH, Troup JP, Witzmann FA, Holloszy JO. The effect of ageing and exercise on skeletal muscle function. Mech Ageing Dev. 1984;27:161–172. doi: 10.1016/0047-6374(84)90041-1. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-L. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol. 1996;490:529–536. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E. Skeletal muscle. In: Mountcastle VB, editor. Medical physiology. 14. St. Louis: Mosby; 1980. pp. 674–702. [Google Scholar]
- Herbert RD, Gandevia SC. Twitch interpolation in human muscles: mechanisms and implications for measurement of voluntary activation. J Neurophysiol. 1999;82:2271–2283. doi: 10.1152/jn.1999.82.5.2271. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Butler JE, Todd G, Gandevia SC, Taylor JL. Supraspinal fatigue does not explain the sex difference in muscle fatigue of maximal contractions. J Appl Physiol. 2006;101:1036–1044. doi: 10.1152/japplphysiol.00103.2006. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Thompson MW, Ruell PA, Harmer AR, Thom JM, Gwinn TH, Adams RD. Human skeletal sarcoplasmic reticulum Ca2+ uptake and muscle function with aging and strength training. J Appl Physiol. 1999;86:1858–1865. doi: 10.1152/jappl.1999.86.6.1858. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Todd G, Butler JE, Gandevia SC, Taylor JL. Recovery from supraspinal fatigue is slowed in old adults after fatiguing maximal isometric contractions. J Appl Physiol. 2008;105:1199–1209. doi: 10.1152/japplphysiol.01246.2007. [DOI] [PubMed] [Google Scholar]
- Jakobi JM, Rice CL. Voluntary muscle activation varies with age and muscle group. J Appl Physiol. 2002;93:457–462. doi: 10.1152/japplphysiol.00012.2002. [DOI] [PubMed] [Google Scholar]
- Jennekens FG, Tomlinson BE, Walton JN. The sizes of the two main histochemical fibre types in five limb muscles in man. An autopsy study. J Neurol Sci. 1971;13:281–292. doi: 10.1016/0022-510X(71)90033-5. [DOI] [PubMed] [Google Scholar]
- Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol. 1990;45:M82–M88. doi: 10.1093/geronj/45.3.M82. [DOI] [PubMed] [Google Scholar]
- Klass M, Baudry S, Duchateau J. Voluntary activation during maximal contraction with advancing age: a brief review. Eur J Appl Physiol. 2007;100:543–551. doi: 10.1007/s00421-006-0205-x. [DOI] [PubMed] [Google Scholar]
- Klein CS, Marsh GD, Petrella RJ, Rice CL. Muscle fiber number in the biceps brachii muscle of young and old men. Muscle Nerve. 2003;28:62–68. doi: 10.1002/mus.10386. [DOI] [PubMed] [Google Scholar]
- Klein CS, Rice CL, Marsh GD. Normalized force, activation, and coactivation in the arm muscles of young and old men. J Appl Physiol. 2001;91:1341–1349. doi: 10.1152/jappl.2001.91.3.1341. [DOI] [PubMed] [Google Scholar]
- Kleine BU, Stegeman DF. Stimulating motor wisdom. J Appl Physiol. 2007;102:1737–1738. doi: 10.1152/japplphysiol.00113.2007. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Mantoni M, Schiaffino S, Ausoni S, Gorza L, Laurent-Winter C, Schnohr P, Saltin B. Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand. 1990;140:41–54. doi: 10.1111/j.1748-1716.1990.tb08974.x. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B. Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand. 1990;140:55–62. doi: 10.1111/j.1748-1716.1990.tb08975.x. [DOI] [PubMed] [Google Scholar]
- Lee M, Gandevia SC, Carroll TJ. Cortical voluntary activation can be reliably measured in human wrist extensors using transcranial magnetic stimulation. Clin Neurophysiol. 2008;119:1130–1138. doi: 10.1016/j.clinph.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Mattiello-Sverzut AC, Chimelli L, Moura MS, Teixeira S, de Oliveira JA. The effects of aging on biceps brachii muscle fibers: a morphometrical study from biopsies and autopsies. Arq Neuropsiquiatr. 2003;61:555–560. doi: 10.1590/S0004-282X2003000400006. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Vandervoort AA, Rice CL. Peripheral impairments cause a progressive age-related loss of strength and velocity-dependent power in the dorsiflexors. J Appl Physiol. 2007;102:1962–1968. doi: 10.1152/japplphysiol.01166.2006. [DOI] [PubMed] [Google Scholar]
- Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123:553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AE, MacDougall JD, Tarnopolsky MA, Sale DG. Gender differences in strength and muscle fiber characteristics. Eur J Appl Physiol Occup Physiol. 1993;66:254–262. doi: 10.1007/BF00235103. [DOI] [PubMed] [Google Scholar]
- Monemi M, Eriksson PO, Eriksson A, Thornell LE. Adverse changes in fibre type composition of the human masseter versus biceps brachii muscle during aging. J Neurol Sci. 1998;154:35–48. doi: 10.1016/S0022-510X(97)00208-6. [DOI] [PubMed] [Google Scholar]
- Monemi M, Eriksson PO, Kadi F, Butler-Browne GS, Thornell LE. Opposite changes in myosin heavy chain composition of human masseter and biceps brachii muscles during aging. J Muscle Res Cell Motil. 1999;20:351–361. doi: 10.1023/A:1005421604314. [DOI] [PubMed] [Google Scholar]
- Nygaard E, Houston M, Suzuki Y, Jorgensen K, Saltin B. Morphology of the brachial biceps muscle and elbow flexion in man. Acta Physiol Scand. 1983;117:287–292. doi: 10.1111/j.1748-1716.1983.tb07208.x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Bentley DJ, Carroll TJ. Locomotor exercise induces long-lasting impairments in the capacity of the human motor cortex to voluntarily activate knee extensor muscles. J Appl Physiol. 2009;106:556–565. doi: 10.1152/japplphysiol.90911.2008. [DOI] [PubMed] [Google Scholar]
- Simunic B, Degens H, Rittweger J, Narici M, Mekjavic IB, Pisot R. Noninvasive estimation of myosin heavy chain composition in human skeletal muscle. Med Sci Sports Exerc. 2011;43:1619–1625. doi: 10.1249/MSS.0b013e31821522d0. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Allen GM, Butler JE, Gandevia SC. Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J Appl Physiol. 2000;89:305–313. doi: 10.1152/jappl.2000.89.1.305. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. Transcranial magnetic stimulation and human muscle fatigue. Muscle Nerve. 2001;24:18–29. doi: 10.1002/1097-4598(200101)24:1<18::AID-MUS2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Todd G, Butler JE, Taylor JL, Gandevia SC. Hyperthermia: a failure of the motor cortex and the muscle. J Physiol. 2005;563:621–631. doi: 10.1113/jphysiol.2004.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Butler JE, Martin PG, Gorman RB, Gandevia SC. Use of motor cortex stimulation to measure simultaneously the changes in dynamic muscle properties and voluntary activation in human muscles. J Appl Physiol. 2007;102:1756–1766. doi: 10.1152/japplphysiol.00962.2006. [DOI] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Gandevia SC. Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J Physiol. 2003;551:661–671. doi: 10.1113/jphysiol.2003.044099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Gandevia SC. Reproducible measurement of voluntary activation of human elbow flexors with motor cortical stimulation. J Appl Physiol. 2004;97:236–242. doi: 10.1152/japplphysiol.01336.2003. [DOI] [PubMed] [Google Scholar]
- van Duinen H, Gandevia SC, Taylor JL. Voluntary activation of the different compartments of the flexor digitorum profundus. J Neurophysiol. 2010;104:3213–3221. doi: 10.1152/jn.00470.2010. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol. 1986;61:361–367. doi: 10.1152/jappl.1986.61.1.361. [DOI] [PubMed] [Google Scholar]
- Yoon T, De-Lap BS, Griffith EE, Hunter SK. Age-related muscle fatigue after a low-force fatiguing contraction is explained by central fatigue. Muscle Nerve. 2008;37:457–466. doi: 10.1002/mus.20969. [DOI] [PubMed] [Google Scholar]
- Yue GH, Ranganathan VK, Siemionow V, Liu JZ, Sahgal V. Older adults exhibit a reduced ability to fully activate their biceps brachii muscle. J Gerontol A Biol Sci Med Sci. 1999;54:M249–M253. doi: 10.1093/gerona/54.5.M249. [DOI] [PubMed] [Google Scholar]