Fig. 2.
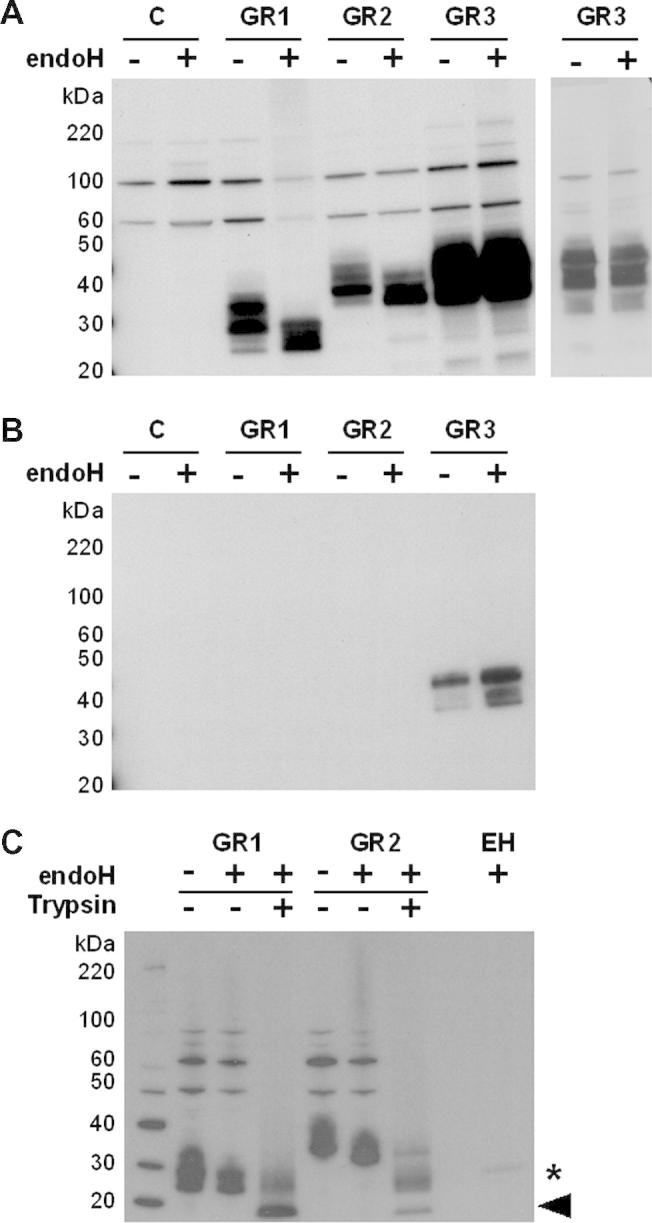
Intracellular forms of the N-glycosylation reporters in C. albicans. Western blots of intracellular extracts of wild type C. albicans cells (CAI4) transformed with pACT-GR1, pACT-GR2, pACT-GR3 or the empty CIp10 vector (C, control) (Table 1). Extracts were either untreated (−) or treated (+) with endoglycosidase H before analysis. (A) Western blots were probed with a polyclonal anti-FLAG antibody. In the left panel, 15 μg of protein sample was loaded per lane. The right panel shows a separate gel containing less protein (5 μg) for the GR3 protein extracts. (B) An analogous western blot probed with a monoclonal anti-His6 antibody. (C) Western blot of trypsin digested intracellular extracts from wild type C. albicans cells (CAI4) expressing pACT-GR1 or pACT-GR2 and probed with anti-FLAG antibody. The carboxy-terminal FLAG-tagged GR tryptic peptides of about 15 kDa are highlighted (arrow) as well as the endoglycosidase H band at about 29 kDa (asterisk). The Coomassie stained gel confirming the protein loading is shown in the supplementary data (Fig. S1).
