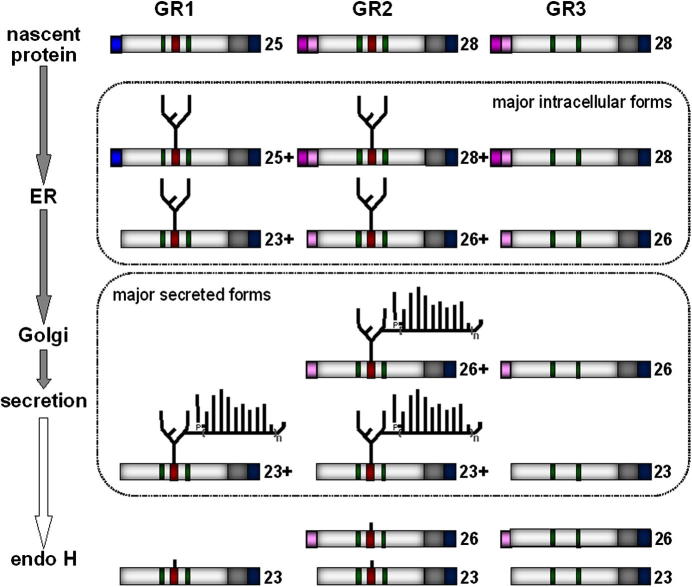
Fig. 6.
Cartoon summarising the observed forms of the GR1, GR2 and GR3 reporters in C. albicans. Each bar represents the structure of a GR protein. (Refer to Fig. 1 for the elements in each protein.) The number to the right of each bar indicates the estimated molecular mass, where + indicates apparent increased mass due to glycosylation. The nascent GR1 and GR2 proteins undergo core glycosylation, presumably in the ER, and a proportion of these proteins have their signal sequences removed (Fig. 2). Note that GR3 lacks the consensus N-glycosylation site and is not glycosylated (Fig. 2). The elaboration of the outer chains on GR1 and GR2 occurs, presumably in the Golgi apparatus, in an Och1 and Pmr1 dependent manner (Figs. 4 and 5). Also, the Sap2 pro-region is cleaved from a proportion of the GR2 and GR3 proteins. These various forms of GR protein are then secreted (Figs. 3–5). Subsequent endoglycosydase H treatment deglycosylates these GR proteins (Figs. 3, 4B and 5B). The major forms of each GR protein observed in the intracellular and extracellular fractions are highlighted by the boxes.