Abstract
Fusarium proliferatum and F. verticillioides are considered as minor pathogens of pea (Pisum sativum L.). Both species can survive in seed material without visible disease symptoms, but still contaminating it with fumonisins. Two populations of pea-derived F. proliferatum and F. verticillioides strains were subjected to FUM1 sequence divergence analysis, forming a distinct group when compared to the collection strains originating from different host species. Furthermore, the mycotoxigenic abilities of those strains were evaluated on the basis of in planta and in vitro fumonisin biosynthesis. No differences were observed in fumonisin B (FB) levels measured in pea seeds (maximum level reached 1.5 μg g−1); however, in rice cultures, the majority of F. proliferatum genotypes produced higher amounts of FB1–FB3 than F. verticillioides strains.
Keywords: FUM cluster, fumonisins, Fusarium proliferatum, Fusarium verticillioides, pea seeds, phylogenetic analysis
1. Introduction
Pea (Pisum sativum L.) is one of the major legume crops, which are bred mainly for their high content of proteins present in pea seeds [1] and their valuable amino acid composition. Fungal diseases are frequently occurring factors limiting yield and quality of seed used for food and feed. Still, Fusarium pathogens are considered to have minor significance [2,3], while Ascochyta sp. and Alternaria sp. play major roles in this field [4,5,6]. However, Fusarium proliferatum and F. verticillioides should not be overlooked as both species are able to produce efficiently a group of the most dangerous Fusarium mycotoxins—fumonisins [7,8,9,10]. They are a family of polyketide derivatives, structurally related to sphinganine, compounds disrupting sphingolipid metabolism, causing different toxicological effects in humans, animals, as well as plants [7]. The most abundant fumonisin produced in nature is fumonisin B1 (FB1), a suspected risk factor for esophageal [11] and liver [12] cancers, neural tube defects [13] and cardiovascular problems [14]. Taking into consideration the available toxicological evidence, the International Agency for Research on Cancer classified FB1 as probably carcinogenic to humans (class 2B carcinogen) [15].
Numerous studies have confirmed the presence of fumonisins in plant material contaminated with their producers, vast majority was focused on maize [16,17,18,19]. However, rice and sorghum are also often infected with Fusaria belonging to the Gibberella fujikuroi species complex: F. fujikuroi, F. proliferatum, F. verticillioides and F. andiyazi [20,21]. Several reports describing the contamination of crop plants with fumonisin-producing Fusarium species included wheat [22,23], garlic [24,25,26,27], asparagus [26,28,29,30,31], pineapple [26,32] and soybean [33].
Until now, little information was provided on pea plants serving as hosts for fumonisin producers and a potential serious threat to human health posed by the contamination of seeds. On the contrary, F. oxysporum, F. solani, F. avenaceum and F. poae have been considered as major pathogens of this crop [2]. None of these species is capable of synthesizing fumonisins [7,9]. Many recent studies were concentrated on the variability of F. proliferatum and F. verticillioides populations occurring in the environment, especially in the geographical and ecological context [8,34,35,36,37]. The latest findings suggest that mycotoxin biosynthetic genes represent good targets to design molecular tools for evolutionary and phylogenetic research with the essential genes from the trichothecene, fumonisin and enniatin/beauvericin metabolic pathways being exploited particularly frequently [26,28,38,39,40,41,42]. Genes encoding the zearalenone and bikaverin pathways are also gaining more attention [41,43]. The high level of sequence divergence among mycotoxin biosynthetic genes (especially TRI and FUM genes) can be applied to distinguish the populations even on a sub-specific (or even host-specific) level [26], being often more valuable phylogenetic markers for the evaluation of the Fusarium species diversity.
The main aim of this study was to analyze the sequence divergences of the translation elongation factor 1 alpha (tef-1α) and FUM1 genes (encoding the essential enzyme of the fumonisin biosynthetic pathway—polyketide synthase) in two populations of F. proliferatum and F. verticillioides originating from several Polish pea varieties, compared to the collection strains of both species obtained from different host species. Moreover, the abilities of the selected strains to produce fumonisins in vitro were evaluated together with the contamination of the pea seed material with those mycotoxins.
2. Results
Seeds of twelve cultivars of pea were screened for presence of the pathogenic fungi. Each genotype was grown in four replicates and two distinct localities in Central Poland: Radzików and Wiatrowo. Fungal species were identified morphologically using optical microscope and only samples containing Fusarium species were included in the study. Two cultivars appeared to contain fumonisin-producing Fusaria in more than one replicate and in both localities: EZOP and TURNIA. Furthermore, several other cultivars also contained the species of interest: EUREKA, SOKOLIK, TARCHALSKA and WIATO. F. proliferatum was predominantly occurring on cv. TURNIA and F. verticillioides on cv. EZOP, EUREKA and TURNIA (Table 1). Apart from both studied species, F. poae, F. equiseti, F. acuminatum, F. avenaceum, F. graminearum and F. sporotrichioides were identified occasionally in plant tissues (results not shown).
Table 1.
Strains of F. proliferatum and F. verticillioiedes purified from pea seedsof different cultivars grown in 2011 in two localities in Poland (R: Radzików, W: Wiatrowo), as well as collection strains used in the phylogenetic analyses.
| Strain | Species | Host/cultivar/locality | Year | Origin |
| KF 3758 | F. proliferatum | P. sativum/SOKOLIK/W | 2012 | Poland |
| KF 3759 | F. proliferatum | P. sativum/TARCHALSKA/W | 2012 | Poland |
| KF 3735 | F. proliferatum | P. sativum/TURNIA/R | 2012 | Poland |
| KF 3736 | F. proliferatum | P. sativum/TURNIA/R | 2012 | Poland |
| KF 3737 | F. proliferatum | P. sativum/TURNIA/R | 2012 | Poland |
| KF 3738 | F. proliferatum | P. sativum/TURNIA/R | 2012 | Poland |
| KF 3731 | F. proliferatum | P. sativum/TURNIA/R | 2012 | Poland |
| KF 3733 | F. proliferatum | P. sativum/TURNIA/R | 2012 | Poland |
| KF 3734 | F. proliferatum | P. sativum/TURNIA/R | 2012 | Poland |
| KF 3763 | F. verticillioides | P. sativum/WIATO/W | 2012 | Poland |
| KF 3764 | F. verticillioides | P. sativum/WIATO/W | 2012 | Poland |
| KF 3765 | F. verticillioides | P. sativum/WIATO/W | 2012 | Poland |
| KF 3661 | F. verticillioides | P. sativum/EUREKA/R | 2012 | Poland |
| KF 3740 | F. verticillioides | P. sativum/EUREKA/R | 2012 | Poland |
| KF 3660 | F. verticillioides | P. sativum/EUREKA/R | 2012 | Poland |
| KF 3766 | F. verticillioides | P. sativum/EZOP/W | 2012 | Poland |
| KF 3767 | F. verticillioides | P. sativum/EZOP/W | 2012 | Poland |
| KF 3768 | F. verticillioides | P. sativum/EZOP/W | 2012 | Poland |
| KF 3769 | F. verticillioides | P. sativum/EZOP/W | 2012 | Poland |
| KF 3770 | F. verticillioides | P. sativum/EZOP/W | 2012 | Poland |
| KF 3771 | F. verticillioides | P. sativum/EZOP/W | 2012 | Poland |
| KF 3772 | F. verticillioides | P. sativum/EZOP/W | 2012 | Poland |
| KF 3773 | F. verticillioides | P. sativum/EZOP/W | 2012 | Poland |
| KF 3774 | F. verticillioides | P. sativum/EZOP/W | 2012 | Poland |
| KF 3775 | F. verticillioides | P. sativum/EZOP/W | 2012 | Poland |
| KF 3776 | F. verticillioides | P. sativum/EZOP/W | 2012 | Poland |
| KF 3778 | F. verticillioides | P. sativum/TARCHALSKA/W | 2012 | Poland |
| KF 3760 | F. verticillioides | P. sativum/TURNIA/W | 2012 | Poland |
| KF 3761 | F. verticillioides | P. sativum/TURNIA/W | 2012 | Poland |
| KF 3781 | F. verticillioides | P. sativum/TURNIA/W | 2012 | Poland |
| KF 3782 | F. verticillioides | P. sativum/TURNIA/W | 2012 | Poland |
| Strain | Species | Collection strains | Year | Origin |
| CBS 513.88 | A. niger | NT_166526.1 | ||
| FOXG_03515 | F. oxysporum | |||
| FVEG_02381 | F. verticillioides | Z. mays | ||
| KF 497 | F. proliferatum | T. aestivum | 1987 | Portugal |
| KF 925 | F. proliferatum | Z. mays | 1986 | Poland |
| KF 3441 | F. proliferatum | Z. mays | 2006 | Poland |
| KF 3654 | F. proliferatum | Z. mays | 2011 | Poland |
| KF 3616 | F. proliferatum | A. cepa | 2011 | Poland |
| KF 3385 | F. proliferatum | A. comosus | 2009 | Vietnam |
| KF 3548 | F. proliferatum | A. comosus | 2011 | Ecuador |
| KF 3550 | F. proliferatum | A. comosus | 2011 | Ecuador |
| KF 3355 | F. proliferatum | A. officinalis | 2009 | Poland |
| KF 3357 | F. proliferatum | A. officinalis | 2009 | Poland |
| KF 3362 | F. proliferatum | A. officinalis | 2009 | Poland |
| KF 3360 | F. proliferatum | A. officinalis | 2009 | Poland |
| KF 3369 | F. proliferatum | A. sativum | 2009 | Poland |
| KF 3372 | F. proliferatum | A. sativum | 2009 | Poland |
| KF 3503 | F. proliferatum | A. sativum | 2010 | Poland |
| KF 3409 | F. proliferatum | Cambria | 2010 | |
| KF 422 | F. proliferatum | O. sativa | 1973 | Taiwan |
| KF 1329 | F. proliferatum | O. sativa | Japan | |
| KF 3584 | F. proliferatum | O. sativa | 2011 | Thailand |
| KF 3416 | F. proliferatum | P. dactylifera | 2010 | Tunisia |
| KF 3321 | F. temperatum | A. comosus | 2008 | Costa Rica |
| KF 3488 | F. verticillioides | Z. mays | 2010 | Poland |
| KF 3482 | F. verticillioides | Z. mays | 2010 | Poland |
| KF 3483 | F. verticillioides | Z. mays | 2010 | Poland |
| KF 3644 | F. verticillioides | Z. mays | 2010 | Poland |
| KF 3537 | F. verticillioides | A. comosus | 2010 | Costa Rica |
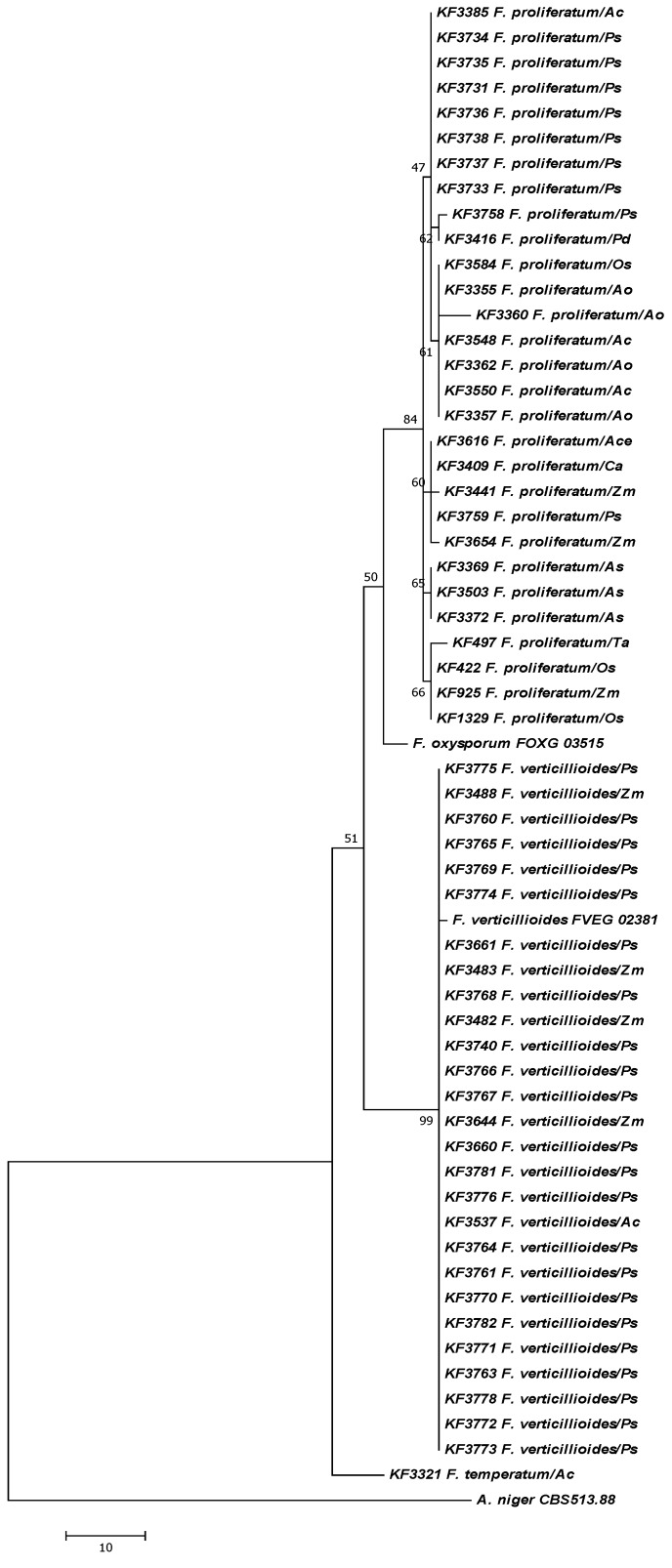
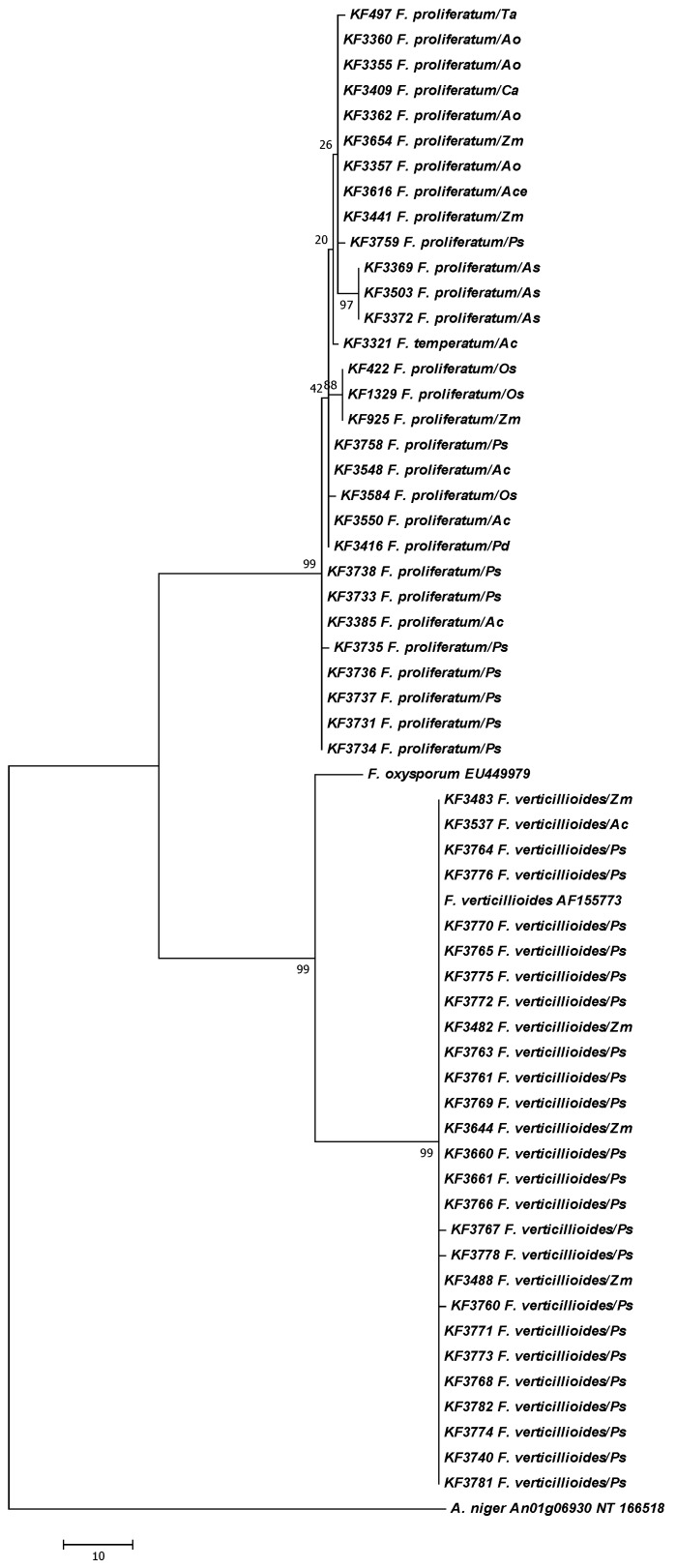
All isolates were re-identified molecularly on the basis of the translation elongation factor 1alpha (tef-1α) sequence analysis and aligned to the sequences of the collection strains from different host species to evaluate the in-population genetic variability (Figure 1). Additionally, a fragment of a FUM1 gene was partially sequenced using primers developed and validated during previous works [26,40]. Based on the multiple alignment of the sequences obtained, a dendrogram was calculated using the Maximum Parsimony approach (Figure 2). All strains under study fell firmly into the clades of F. proliferatum and F. verticillioides, discriminated on the basis of the collection strains sequences (Table 1). Moreover, a certain level of sub-specific polymorphism has been observed among the strains of F. proliferatum (Figure 1, Figure 2).
Figure 1.
The most parsimonious tree for 57 Fusarium strains used in the study based on the translation elongation factor 1alpha (tef-1α) sequences. The reference strains of F. oxysporum (FOXG_03515) and F. verticillioides (FVEG_02381), as well as an outgroup of A. niger CBS 513.88 strain (GenBank Acc. NT_166526.1) were included (Fungal Genome Initiative). Maximum Parsimony approach and bootstrap test (1000 replicates) were applied. Abbreviations used for the host names: Ac: Ananas comosus; Ace: Allium cepa; Ao: Asparagus officinalis; As: Allium sativum; Ca: Cambria sp.; Os: Oryza sativa; Pd: Phoenix dactylifera; Ps: Pisum sativum; Ta: Triticum aestivum; Zm: Zea mays.
Figure 2.
The most parsimonious tree for 57 Fusarium strains used in the study based on the partial sequence of the FUM1 gene. The reference strains of F. oxysporum (GenBank Acc. EU449979) and F. verticillioides (GenBank Acc. AF155773), as well as an outgroup of A. niger CBS 513.88 strain (GenBank Acc. NT_166526.1) were included). Maximum Parsimony approach and bootstrap test (1000 replicates) were applied. Abbreviations used for the host names: Ac: Ananas comosus; Ace: Allium cepa; Ao: Asparagus officinalis; As: Allium sativum; Ca: Cambria sp.; Os: Oryza sativa; Pd: Phoenix dactylifera; Ps: Pisum sativum; Ta: Triticum aestivum; Zm: Zea mays.
Having uncovered that pea seed samples quite frequently carried the dangerous Fusarium pathogens, a survey for fumonisin quantification has been performed for the respective seed samples of two cultivars EUREKA and TURNIA (four replicates from two localities each). Cultivars were chosen on the basis of fungal species present (Table 1). All of the samples tested contained low amounts of FBs (Table 2). However, no significant differences have been observed in FB levels between seeds containing F. proliferatum and F. verticillioides as the prevailing pathogens. A maximum amount of FBs detected in TURNIA IV from Radzików was 1.72 μg g−1, and the lowest (for TURNIA I from Radzików) was 0.63 μg g−1. FB1 was dominating markedly, representing more than 90% of the total amount at all times (Table 2).
Table 2.
Fumonisin concentration (in μg g−1) and standard deviations (SD) in seeds of two pea cultivars (EUREKA and TURNIA) grown in 2011 season in two distinct localities of Poland (R: Radzików, W: Wiatrowo) and naturally infected with fumonisin-producing F. verticillioides and F. proliferatum.
| Sample | FB1 | FB2 | FB3 |
|---|---|---|---|
| EUREKA_I (W) | 1.12 | 0.05 | 0.01 |
| EUREKA _II (W) | 1.34 | 0.07 | 0.02 |
| EUREKA _III (W) | 0.79 | 0.05 | 0.01 |
| EUREKA _IV (W) | 0.81 | 0.04 | 0.01 |
| Mean ± SD | 1.02 ± 0.26 | 0.05 ± 0.01 | 0.01 ± 0.01 |
| EUREKA_I (R) | 1.11 | 0.12 | 0.05 |
| EUREKA_II (R) | 0.72 | 0,03 | 0.00 |
| EUREKA III (R) | 0.45 | 0.12 | 0.08 |
| EUREKA_IV (R) | 0.63 | 0.04 | 0.01 |
| Mean ± SD | 0.73 ± 0.28 | 0.08 ± 0.05 | 0.04 ± 0.04 |
| TURNIA_I (W) | 0.85 | 0.11 | 0.04 |
| TURNIA _II (W) | 0.81 | 0.09 | 0.05 |
| TURNIA _III (W) | 0.92 | 0.07 | 0.01 |
| TURNIA _IV (W) | 0.91 | 0.08 | 0.02 |
| Mean ± SD | 0.87 ± 0.05 | 0.09 ± 0.02 | 0.03 ± 0.02 |
| TURNIA_I (R) | 0.55 | 0.06 | 0.02 |
| TURNIA_II (R) | 0.61 | 0.07 | 0.01 |
| TURNIA_III (R) | 1.27 | 0.14 | 0.08 |
| TURNIA_IV (R) | 1.48 | 0.15 | 0.09 |
| Mean ± SD | 0.98 ± 0.47 | 0.11 ± 0.05 | 0.05 ± 0.04 |
In order to evaluate the efficacy of the fumonisin B1–B3 biosynthesis by F. proliferatum and F. verticillioides isolates, the in vitro cultures on sterile rice grain were prepared [40]. Eighteen genotypes originating from TURNIA, EUREKA and SOKOLIK cultivars were analyzed. The amounts of FBs were quantified using a standardized high-performance liquid chromatography (HPLC) method (Section 4.5). Additionally, several collection strains of F. proliferatum and F. verticillioides originating from various host species [26] have been included to show the intraspecific variability of this trait (Table 3).
Table 3.
Means and standard deviations (SD) of fumonisins concentrations (in μg g−1) produced in rice cultures by F. verticillioides and F. proliferatum strains purified from infected seeds of various pea cultivars from two localities (R: Radzików, W: Wiatrowo), as well as by several collection strains of both species (please refer to [26] for more F. proliferatum strains).
| Strain | Species | Host/cultivar/locality | FB1 | FB2 | FB3 |
|---|---|---|---|---|---|
| KF 3779 | F. verticillioides | P. sativum/TURNIA/W | 155.92 ± 12.31 | 0.60 ± 0.02 | 5.44 ± 1.13 |
| KF 3780 | F. verticillioides | P. sativum/TURNIA/W | 183.55 ± 14.03 | 0.98 ± 0.03 | 16.75 ± 2.52 |
| KF 3760 | F. verticillioides | P. sativum/TURNIA/W | 317.24 ± 16.32 | 34.00 ± 3.15 | 7.01 ± 1.14 |
| KF 3781 | F. verticillioides | P. sativum/TURNIA/W | 80.39 ± 7.44 | 0.49 ± 0.15 | 2.66 ± 0.09 |
| KF 3761 | F. verticillioides | P. sativum/TURNIA/W | 202.29 ± 10.18 | 95.61 ± 8.53 | 37.44 ± 4.79 |
| KF 3782 | F. verticillioides | P. sativum/TURNIA/W | 111.65 ± 11.59 | 52.35 ± 4.17 | 27.62 ± 5.56 |
| KF 3731 | F. proliferatum | P. sativum/TURNIA/R | 111.07 ± 9.47 | 49.61 ± 5.22 | 43.58 ± 6.85 |
| KF 3732 | F. proliferatum | P. sativum/TURNIA/R | 958.51 ± 21.06 | 271.22 ± 10.84 | 104.30 ± 9.48 |
| KF 3733 | F. proliferatum | P. sativum/TURNIA/R | 121.65 ± 10.11 | 51.99 ± 6.63 | 37.71 ± 5.54 |
| KF 3734 | F. proliferatum | P. sativum/TURNIA/R | 49.36 ± 5.33 | 29.26 ± 4.12 | 30.85 ± 3.52 |
| KF 3735 | F. proliferatum | P. sativum/TURNIA/R | 845.56 ± 42.67 | 227.99 ± 10.47 | 112.65 ± 9.65 |
| KF 3736 | F. proliferatum | P. sativum/TURNIA/R | 476.53 ± 25.13 | 227.78 ± 11.58 | 111.39 ± 8.83 |
| KF 3737 | F. proliferatum | P. sativum/TURNIA/R | 106.09 ± 9.88 | 49.72 ± 5.39 | 43.84 ± 5.28 |
| KF 3738 | F. proliferatum | P. sativum/TURNIA/R | 648.30 ± 21.56 | 180.68 ± 15.41 | 101.13 ± 4.69 |
| KF 3660 | F. verticillioides | P. sativum/EUREKA/R | 78.74 ± 7.40 | 24.20 ± 3.30 | 4.68 ± 0.08 |
| KF 3740 | F. verticillioides | P. sativum/EUREKA/R | 216.50 ±15.84 | 89.95 ± 7.17 | 43.33 ± 6.16 |
| KF 3661 | F. verticillioides | P. sativum/EUREKA/R | 1861.52 ± 54.23 | 108.16 ± 7.49 | 40.61 ± 5.58 |
| KF 3758 | F. proliferatum | P. sativum/EZOP/W | 212.02 ± 12.36 | 63.50 ± 8.82 | 44.01 ± 6.85 |
| KF 3416 | F. proliferatum | P. dactylifera | 46.34 ± 5.41 | 26.11 ± 5.41 | 6.53 ± 0.08 |
| KF 3357 | F. proliferatum | A. officinalis | 1536.00 ± 52.33 | 657.09 ± 80.35 | 123.72 ± 8.71 |
| KF 3654 | F. proliferatum | Z. mays | 1578.04 ± 41.25 | 529.96 ± 69.15 | 91.34 ± 9.13 |
| KF 3584 | F. proliferatum | O. sativa | 201.20 ± 7.69 | 53.60 ± 7.74 | 33.00 ± 4.28 |
| KF 3409 | F. proliferatum | Cambria sp. | 668.72 ± 18.47 | 170.07 ± 25.13 | 70.19 ± 6.71 |
| KF 3503 | F. proliferatum | A. sativum | 1186.87 ± 87.42 | 185.54 ± 18.53 | 66.24 ± 5.93 |
| KF 3537 | F. verticillioides | A. comosus | 59.65 ± 6.06 | 19.37 ± 2.47 | 5.86 ± 0.98 |
| KF 3644 | F. verticillioides | Z. mays | 6.12 ± 1.52 | 0.37 ± 0.05 | 0.29 ± 0.03 |
| KF 3488 | F. verticillioides | Z. mays | 39.77 ± 4.15 | 0.96 ± 0.09 | 0.06 ± 0.01 |
| KF 3482 | F. verticillioides | Z. mays | 273.38 ± 14.39 | 60.35 ± 1.11 | 0.88 ± 0.04 |
| KF 3483 | F. verticillioides | Z. mays | 14.17 ± 2.08 | 0.04 ± 0.01 | 0.00 ± 0.00 |
3. Discussion
Seeds of only six out of twelve pea cultivars screened appeared to contain fumonisin-producing Fusarium species and in the seeds of EZOP and TURNIA the pathogens occurred frequently. F. proliferatum was predominantly occurring on cv. TURNIA and F. verticillioides on cv. EZOP (Table 1). Other Fusarium species were isolated only occasionally. Species identification was performed on the basis of the translation elongation factor 1alpha (tef-1α) sequence analysis. This gene is widely used in phylogenetic studies of fungi, successfully resolving most of the closely related Fusarium species [17,44,45]. However, in populations of some less polymorphic species, where the genotypes studied display a low level of genetic diversity, different genomic regions should be used to increase the polymorphism revealed [38,46].
Polyketide synthase is the essential enzyme of the fumonisin biosynthetic pathway is encoded by FUM1 gene [47]. Using primers developed and validated during the previous works [26,40], FUM1 gene fragments were sequenced and comparatively analyzed using all the strains included in the study. Finally, a dendrogram was calculated using the Maximum Parsimony approach to show the divergences among the strains originating from different host species. All strains formed two separate and well-supported clades of F. proliferatum and F. verticillioides (Figure 2). Furthermore, a certain level of sub-specific polymorphism has been observed among the strains in the case of F. proliferatum genotypes (Figure 2). In the case of FUM genes this observation was already reported [26,40]; however, the analysis of the pea-derived strains is presented here for the first time. It seems that the pea-derived strains of F. proliferatum are highly uniform and show the highest similarity level to some genotypes originating from pineapple and date palm (Figure 2). On the contrary, F. verticillioides strains have shown virtually no difference among the populations from different hosts. It could implicate that F. proliferatum displays a higher evolutionary potential. In fact, this hypothesis seems to be fairly supported by the results of analyses performed during this and the previous studies [8,26,36,40].
Furthermore, the sequences of the biosynthetic genes from other mycotoxin pathways have been utilized in phylogenetic studies of Fusarium species [38,41,42], showing considerably higher polymorphism than the commonly used conserved genes from the primary metabolic pathways. Thus, markers for secondary metabolite biosynthetic genes can be sensitive tools for the prediction of the mycotoxin presence in plant samples. Here, pea seeds of the cultivars containing F. verticillioides have been analyzed. All samples tested contained low amounts of FBs (Table 1), though the levels of FBs were similar in the samples of seeds containing F. proliferatum and F. verticillioides, as the prevailing pathogens. FB1 dominated markedly, representing more than 90% of the total amount at all times (Table 2). Moreover, there was no correlation between the frequency of the pathogen detection in particular pea cultivar samples coming from different locations and the observed fumonisin content. The efficacies of the fumonisin B1–B3 biosynthesis by eighteen genotypes of F. proliferatum and F. verticillioides strains originating from TURNIA, EUREKA and SOKOLIK cultivars, were evaluated on the basis of the in vitro cultures on sterile rice grain [40]. Concentrations of fumonisins produced on this substrate were lower than these observed on maize kernels [16,36], but still exceeded 1.5 mg g−1 for some strains (Table 3). This difference may be related to the starch content of maize grain. Another possible reason is the crucial role of fumonisins during maize infestation, which has not been proven for other host-pathogen systems yet [48]. Generally, in rice cultures F. proliferatum genotypes produced higher amounts of FBs than F. verticillioides strains, however, the most efficient strain was F. verticillioides strain KF3661 from cultivar EUREKA (Table 3). Remarkably, the ratios between FB1, FB2 and FB3 have shown higher variance than in the case of pea seed analyses. Some F. proliferatum genotypes accumulated FB2 in amounts measuring as much as 1/3 of FB1 level (e.g., KF 3357 and KF 3654). Conversely, few F. verticillioides genotypes (KF 3780 and KF 3781) synthesized virtually no FB2 with simultaneous higher amounts of FB3. Similar results were obtained for F. verticillioides strains originating from maize, though, FB2 and FB3 were almost absent there (e.g., KF 3644, KF 3488). In fact, for those incidences also FB1 was produced in very low amounts. Moreover, for several medium-producing strains (e.g., F. proliferatum KF 3731 and KF 3737) the levels of FB2 and FB3 were similar and reached almost a half of the FB1 amounts (Table 3).
4. Experimental Section
4.1. Seed Samples and Purification of Fungal Strains
Twelve pea cultivars (EUREKA, EZOP, GWAREK, HUBAL, LASSO, MEDAL, SANTANA, SOKOLIK, TARCHALSKA, TURNIA, WENUS and WIATO) were grown in two localities in Central Poland (Radzików and Wiatrowo) in 2011 season. Each genotype was sown in four randomly localized replicates, which were subsequently considered as a single sample. Ten cultivars originating from Poland, one from Germany (SANTANA) and one from Belgium (LASSO), registered between 1998 and 2011, were tested for the fungi occurrence. Fifty seeds were surface-sterilized with 0.5% sodium hypochlorite for 30 s, rinsed with sterile water and plated on a water-soaked paper in the sterile Petri-dishes for seven days. After that time seeds infected with filamentous fungi were transferred onto new plates with potato dextrose agar (PDA) medium. Hyphae tips were passaged several times on clean PDA plates to purify the strains, which were then inoculated on the synthetic nutrient agar (SNA) medium for microscopic species identification and also on the PDA plates to collect the mycelia for the extraction of the genomic DNAs.
4.2. Fusarium Species Identification
Only Fusarium-infected seed samples were considered in further analyses. Fusarium species were identified morphologically according to Nelson et al. [49] manual. Optical microscope (Olympus, Tokyo, Japan) and 100× of total magnification was used for observation of the presence of microconidia and the nature of the conidiogenous cells.
4.3. Molecular Analyses: DNA Extraction, Primers and PCR Conditions
Genomic DNA extraction was done using a Cetyltrimethyl Ammonium Bromide (CTAB-based method [50]. Partial sequence of the tef-1α gene was amplified using Ef728M (CATCGAGAAGTTCGAGAAGG)/Tef1R (GCCATCCTTGGAGATACCAGC) primer combination [40]. Fum1F1 (CACATCTGTGGGCGATCC)/Fum1R2 (ATATGGCCCCAGCTGCATA) primers were used for the amplification of FUM1 gene fragments [26,40]. The polymerase chain reaction (PCR) was done in 20 μL aliquots using PTC-200 and C-1000 thermal cyclers (BioRad, Hercules, CA, USA). Each sample contained 1 unit of Phire II HotStart Taq DNA polymerase (Finnzymes, Espoo, Finland), 4 μL of 10× PCR buffer, 12.5 pmol of forward/reverse primers, 2.5 mM of each dNTP and about 20–50 ng of fungal DNA. PCR conditions were as follows: 30 s at 98 °C, 35 cycles of (5 s at 98 °C, 5 s at 63 °C, 15 s at 72 °C) and 1 min at 72 °C. Amplicons were electrophoresed in 1.5% agarose gels (Invitrogen, Carlsbad, CA, USA) with ethidium bromide.
4.4. DNA Sequencing, Analysis and Phylogeny Reconstruction
PCR-amplified DNA fragments were purified for sequence analysis with exonuclease I (Epicentre, Madison, WI, USA) and shrimp alkaline phosphatase (Promega, Madison, WI, USA) using the following program: 30 min at 37 °C, followed by 15 min at 80 °C. Both strands were labeled using the BigDyeTerminator 3.1 kit (Applied Biosystems, Foster City, CA, USA), according to Błaszczyk et al. [51] and the manufacturer’s instructions. Labeled fragments were precipitated with ethanol to remove the remains of the reagents. Sequence reading was performed using Applied Biosystems equipment.
Sequences were compared to the NCBI GenBank-deposited sequences to confirm the correct morphological species identification using BLASTn algorithm (MEGABLAST). The collection strains of F. proliferatum and F. verticillioides originating from different host species were included for comparative analysis (Table 3).
The sequences of the PCR products were aligned with ClustalW algorithm. Phylogenetic relationships were reconstructed with MEGA4 software package [52] using Maximum Parsimony approach (Closest Neighbor Interchange heuristics). No gap-containing positions were considered in phylogeny analysis. All reconstructions were tested by bootstrapping with 1000 replicates.
4.5. Fumonisin Quantification
Ten dried pea seeds of each sample (about 5.5 g in weight) were ground using a steel ball mill (Tissue Lyser II). Homogenized plant material was then subjected to the fumonisin extraction procedure (see below).
For toxin quantification rice cultures were prepared for individual Fusarium isolates [42]. Long-grain white rice samples were used (50 g per flask with the addition of 12.5 mL of sterile water), left overnight and sterilized by autoclaving the next day. The rice samples were subsequently inoculated with 4 cm2 of 7-day-old mycelium on potato dextrose agar (PDA) medium. Culture humidity was kept around 30% for 14 days. Then the cultures were dried in room temperature.
Standards of pure FB1, FB2 and FB3; (Sigma, St. Louis, MO, USA). Acetonitrile, methanol (HPLC grade), disodium tetraborate, 2-mercaptoethanol were purchased from Sigma-Aldrich. Potassium hydroxide, acetic acid, o-phosphoric acid were purchased from POCh (Gliwice, Poland). Water for the HPLC mobile phase was purified using a Milli-Q system (Millipore, Bedford, MA, USA).
Samples (5 g) of plant material were homogenized for 3 min in 10 mL of methanol-water (3:1, v/v) and filtered through Whatman No. 4 filter paper. The extract was adjusted to pH 5.8–6.3 using 0.1 mol L−1 KOH. A SAX cartridge was attached to the solid-phase extraction (SPE) manifold unit (Supelco, Bellefonte, PA, USA), following the method described by Waśkiewicz et al. [30]. The o-phosphoric acid (OPA) reagent (20 mg per 0.5 mL of methanol) was prepared and diluted with 2.5 mL of 0.1 mol L−1 disodium tetraborate (Na2B4O7 × 10 H2O). It was then combined with 25 μL 2-mercaptoethanol, which was added to the solution. The FBs standards (5 μL) or extracts (20 μL) were derivatized with 20 μL or 80 μL of the OPA reagent. The reaction mixture (10 μL) was injected onto an HPLC column 3 min later. After filtration through a 0.45 μm Waters HV membrane, methanol-sodium dihydrogen phosphate (0.1 mol L−1 in water) solution (77:23, v/v), adjusted to pH 3.35 with o-phosphoric acid, was used as a mobile phase with a flow rate of 0.6 mL min−1.
A Waters 2695 HPLC instrument (Waters Division of Millipore, Milford, MA, USA) with an X-Bridge column (3.9 mm × 100 mm) and a Waters 2475 fluorescence detector (λEX = 335 nm, λEM = 440 nm) were used for determining the quantity of metabolites. The detection limit was 10 ng g−1 for FBs. Positive results (on the basis of retention time) were confirmed by HPLC analysis of standards and compared with the relevant calibration curves (correlation coefficients for FB1, FB2 and FB3 were 0.9987, 0.9991 and 0.9979, respectively). Recoveries for fumonisins were 94%, 98% and 89%, respectively, which were measured in triplicate by extracting the mycotoxins from blank samples spiked with 10–100 ng g−1 of the compound. The relative standard deviations (RSD) were below 7%.
5. Conclusions
It can be concluded that the pea-originating F. proliferatum and F. verticillioides isolates produced less fumonisins than the genotypes originating from different host species, like maize, garlic or asparagus [8,26,38]. Also, some pineapple-derived F. proliferatum strains were found to be very efficient FB-producers [33]. Comparing the genetic diversity of the two species, F. verticillioides appears as more uniform, but still, the strains differed remarkably in FBs synthesis. Taking into account the divergence of the FUM1 gene in relation to the variance observed in the amounts of FBs produced in vitro, it is the differential regulation pattern governing this variance, rather than the structural divergences of the essential fumonisin biosynthetic genes. This hypothesis, however, needs to be confirmed by conducting additional experiments, e.g., by analyzing the transcription levels of the essential FUM genes.
Acknowledgments
The study was supported by the PMSHE Project NN310 732440, as well as by the Polish Council of Ministers, National Multi-Year Project 2011-2015 (the Resolution number 149/2011, RM 111-138-11).
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Urbano G., Aranda P., Gómez-Villalva E., Frejnagel S., Porres J.M., Frías J., Vidal-Valverde C.N., López-Jurado M. Nutritional evaluation of Pea (Pisum sativum L.) protein diets after mild hydrothermal treatment and with and without added phytase. J. Agric. Food Chem. 2003;51:2415–2420. doi: 10.1021/jf0209239. [DOI] [PubMed] [Google Scholar]
- 2.Marcinkowska J. Fungi occurrence on seeds of field pea. Acta Mycol. 2008;43:77–89. [Google Scholar]
- 3.Ozgonen H., Gulcu M. Determination of mycoflora of pea (Pisum sativum) seeds and the effects of Rhizobium leguminosorum on fungal pathogens of peas. Afr. J. Biotechnol. 2011;10:6235–6240. [Google Scholar]
- 4.Ali M., Nitschke L., Krause M., Cameron B. Selection of pea lines for resistance to pathotypes of Ascochyta pinodes, A. pisi and Phoma medicaginis var. pinodella. Aust. J. Agric. Res. 1978;29:841–849. [Google Scholar]
- 5.Bretag T.W., Keane P.J., Price T.V. The epidemiology and control of ascochyta blight in field peas: A review. Aust. J. Agric. Res. 2006;57:883–902. doi: 10.1071/AR05222. [DOI] [Google Scholar]
- 6.Susuri L., Hagedorn D.J., Rand R.E. Alternaria blight of pea. Plant. Dis. 1982;66:328–330. doi: 10.1094/PD-66-328. [DOI] [Google Scholar]
- 7.Desjardins A.E. Fusarium Mycotoxins: Chemistry, Genetics and Biology. Amer Phytopathological Society; St. Paul, MN, USA: 2006. [Google Scholar]
- 8.Jurado M., Marin P., Callejas C., Moretti A., Vazquez C., Gonzalez-Jaen M.T. Genetic variability and fumonisin production by Fusarium proliferatum. Food Microbiol. 2010;27:50–57. doi: 10.1016/j.fm.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Rheeder J.P., Marasas W.F.O., Vismer H.F. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 2002;68:2101–2105. doi: 10.1128/AEM.68.5.2101-2105.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waśkiewicz A., Beszterda M., Golinski P. Occurrence of fumonisins in food—An interdisciplinary approach to the problem. Food Control. 2012;26:491–499. [Google Scholar]
- 11.Marasas W.F.O. Discovery and occurrence of the fumonisins: A historical perspective. Environ. Health Perspect. 2001;109:239–243. doi: 10.1289/ehp.01109s2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueno Y., Iijima K., Wang S.D., Sugiura Y., Sekijima M., Tanaka T., Chen C., Yu S.Z. Fumonisins as a possible contributory risk factor for primary liver cancer: A 3-year study of corn harvested in Hainan, China by HPLC and ELISA. Food Chem. Toxicol. 1997;35:1143–1150. doi: 10.1016/s0278-6915(97)00113-0. [DOI] [PubMed] [Google Scholar]
- 13.Missmer S.A., Suarez L., Felkner M., Wang E., Merrill A.H., Jr., Rothman K.J., Hendricks K.A. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ. Health Perspect. 2006;114:237–241. doi: 10.1289/ehp.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fincham J.E., Marasas W.F.O., Taljaard J.J., Kriek N.P., Badenhorst C.J., Gelderblom W.C., Seier J.V. Atherogenic effects in a non-human primate of Fusarium moniliforme cultures added to a carbohydrate diet. Atherosclerosis. 1992;94:13–25. doi: 10.1016/0021-9150(92)90183-h. [DOI] [PubMed] [Google Scholar]
- 15.International Agency for Research on Cancer (IARC) Fumonisin B1. IARC Monographs on the Evaluation of the Carcinogenic Risks to Humans: Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. Volume 82. IARC; Lyon, France: 2002. pp. 301–366. [PMC free article] [PubMed] [Google Scholar]
- 16.Covarelli L., Stifano S., Beccari G., Raggi L., Lattanzio V.M.T., Albertini E. Characterization of Fusarium verticillioides strains isolated from maize in Italy: Fumonisin production, pathogenicity and genetic variability. Food Microbiol. 2012;31:17–24. doi: 10.1016/j.fm.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Scauflaire J., Gourgue M., Munaut F. Fusarium temperatum sp. nov. from maize, an emergent species closely related to Fusarium subglutinans. Mycologia. 2011;103:586–597. doi: 10.3852/10-135. [DOI] [PubMed] [Google Scholar]
- 18.Torelli E., Firrao G., Bianchi G., Saccardo F., Locci R. The influence of local factors on the prediction of fumonisin contamination in maize. J. Sci. Food Agric. 2012;92:1808–1814. doi: 10.1002/jsfa.5551. [DOI] [PubMed] [Google Scholar]
- 19.Waśkiewicz A., Wit M., Goliński P., Chełkowski J., Warzecha R., Ochodzki P., Wakuliński W. Kinetics of fumonisin B1 formation in maize ears inoculated with Fusarium verticillioides. Food Addt. Contam. 2012;29:1752–1761. doi: 10.1080/19440049.2012.712061. [DOI] [PubMed] [Google Scholar]
- 20.Hsuan H.M., Salleh B., Zakaria L. Molecular identification of Fusarium species in Gibberella fujikuroi species complex from rice, sugarcane and maize from Peninsular Malaysia. Int. J. Mol. Sci. 2011;12:6722–6732. doi: 10.3390/ijms12106722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma R., Thakur R.P., Senthilvel S., Nayak S., Reddy S.V., Rao V.P., Varshney R.K. Identification and characterization of toxigenic Fusaria associated with sorghum grain mold complex in India. Mycopathologia. 2011;171:223–230. doi: 10.1007/s11046-010-9354-x. [DOI] [PubMed] [Google Scholar]
- 22.Chehri K., Jahromi S.T., Reddy K.R.N., Abbasi S., Salleh B. Occurrence of Fusarium spp. and fumonisins in stored wheat grains marketed in Iran. Toxins. 2010;2:2816–2823. doi: 10.3390/toxins2122816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desjardins A.E., Busman M., Proctor R.H., Stessman R. Wheat kernel black point and fumonisin contamination by Fusarium proliferatum. Food Addit. Contam. 2007;24:1131–1137. doi: 10.1080/02652030701513834. [DOI] [PubMed] [Google Scholar]
- 24.Palmero D., de Cara M., Nosir W., Gálvez L., Cruz A., Woodward S., González-Jaén M.T., Tello J.C. Fusarium proliferatum isolated from garlic in Spain: Identification, toxigenic potential and pathogenicity on related Allium species. Phytopathol. Mediterr. 2012;51:207–218. [Google Scholar]
- 25.Stankovic S., Levic J., Petrovic T., Logrieco A., Moretti A. Pathogenicity and mycotoxin production by Fusarium proliferatum isolated from onion and garlic in Serbia. Eur. J. Plant Pathol. 2007;118:165–172. doi: 10.1007/s10658-007-9126-8. [DOI] [Google Scholar]
- 26.Stępień Ł., Koczyk G., Waśkiewicz A. Genetic and phenotypic variation of Fusarium proliferatum isolates from different host species. J. Appl. Genet. 2011;52:487–96. doi: 10.1007/s13353-011-0059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonti S., Prà M.D., Nipoti P., Prodi A., Alberti I. First report of Fusarium proliferatum causing rot of stored garlic bulbs (Allium sativum L.) in Italy. J. Phytopathol. 2012;160:761–763. doi: 10.1111/jph.12018. [DOI] [Google Scholar]
- 28.Von Bargen S., Martinez O., Schadock I., Eisold A.M., Gossmann M., Buttner C. Genetic variability of phytopathogenic Fusarium proliferatum associated with crown rot in Asparagus officinalis. J. Phytopathol. 2009;157:446–456. doi: 10.1111/j.1439-0434.2008.01525.x. [DOI] [Google Scholar]
- 29.Karolewski Z., Waśkiewicz A., Irzykowska L., Bocianowski J., Kostecki M., Goliński P., Knaflewski M., Weber Z. Fungi presence and their mycotoxins distribution in asparagus spears. Polish J. Environ. Stud. 2011;20:911–919. [Google Scholar]
- 30.Waśkiewicz A., Irzykowska L., Bocianowski J., Karolewski Z., Kostecki M., Weber Z., Goliński P. Occurrence of Fusarium fungi and mycotoxins in marketable asparagus spears. Polish J. Environ. Stud. 2010;49:367–372. [Google Scholar]
- 31.Waśkiewicz A., Irzykowska L., Karolewski Z., Bocianowski J., Kostecki M., Goliński P., Knaflewski M., Weber Z. Fusarium spp. and mycotoxins present in asparagus spears. Cereal Res. Comm. 2008;36:405–407. [Google Scholar]
- 32.Waśkiewicz A., Stępień L. Mycotoxins biosynthesized by plant-derived Fusarium isolates. Arch. Ind. Hyg. Toxicol. 2012;63:479–488. doi: 10.2478/10004-1254-63-2012-2230. [DOI] [PubMed] [Google Scholar]
- 33.Garcia D., Barros G., Chulze S., Ramos A.J., Sanchis V., Marín S. Impact of cycling temperatures on Fusarium verticillioides and Fusarium graminearum growth and mycotoxins production in soybean. J. Sci. Food Agric. 2012;92:2952–2959. doi: 10.1002/jsfa.5707. [DOI] [PubMed] [Google Scholar]
- 34.Punja Z.K., Wan A., Rahman M., Goswami R.S., Barasubiye T., Seifert K.A., Lévesque C.A. Growth, population dynamics, and diversity of Fusarium equiseti in ginseng fields. Eur. J. Plant Pathol. 2008;121:173–184. doi: 10.1007/s10658-007-9261-2. [DOI] [Google Scholar]
- 35.Reynoso M.M., Chulze S.N., Zeller K.A., Torres A.M., Leslie J.F. Genetic structure of Fusarium verticillioides populations isolated from maize in Argentina. Eur. J. Plant. Pathol. 2009;123:207–215. doi: 10.1007/s10658-008-9359-1. [DOI] [Google Scholar]
- 36.Rocha L.O., Reis G.M., da Silva V.N., Braghini R., Teixeira M.M., Corrêa B. Molecular characterization and fumonisin production by Fusarium verticillioides isolated from corn grains of different geographic origins in Brazil. Int. J. Food Microbiol. 2011;145:9–21. doi: 10.1016/j.ijfoodmicro.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Rossi V., Scandolara A., Battilani P. Effect of environmental conditions on spore production by Fusarium verticillioides, the causal agent of maize ear rot. Eur. J. Plant. Pathol. 2009;123:159–169. doi: 10.1007/s10658-008-9351-9. [DOI] [Google Scholar]
- 38.Kulik T., Pszczółkowska A., Łojko M. Multilocus phylogenetics show high intraspecific variability within Fusarium avenaceum. Int. J. Mol. Sci. 2011;12:5626–5640. doi: 10.3390/ijms12095626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Proctor R.H., McCormick S.P., Alexander N.J., Desjardins A.E. Evidence that a secondary metabolite gene cluster has grown by gene relocation during evolution of the filamentous fungus Fusarium. Mol. Microbiol. 2009;74:1128–1142. doi: 10.1111/j.1365-2958.2009.06927.x. [DOI] [PubMed] [Google Scholar]
- 40.Stępień Ł., Koczyk G., Waśkiewicz A. FUM cluster divergence in fumonisins-producing Fusarium species. Fungal Biol. 2011;115:112–123. doi: 10.1016/j.funbio.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Stępień Ł., Gromadzka K., Chełkowski J. Polymorphism of mycotoxin biosynthetic genes among Fusarium equiseti isolates from Italy and Poland. J. Appl. Genet. 2012;53:227–36. doi: 10.1007/s13353-012-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stępień Ł., Waśkiewicz A. Beauvericin and enniatins biosynthesis by Fusarium species and its relation to the divergence of enniatin synthase gene. Toxins. 2013 doi: 10.3390/toxins5030537. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butchko R.A., Brown D.W., Busman M., Tudzynski B., Wiemann P. Lae1 regulates expression of multiple secondary metabolite gene clusters in Fusarium verticillioides. Fungal Genet. Biol. 2012;49:602–612. doi: 10.1016/j.fgb.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Geiser D.M., der mar Jimenez-Gasco M., Kang S., Makalowska I., Veeraraghavan N., Ward T.J., Zhang N., Kuldau G.A., O’Donnell K. FUSARIUM-ID v.1.0: A DNA sequence database for identifying Fusarium. Eur. J. Plant Pathol. 2004;110:473–479. doi: 10.1023/B:EJPP.0000032386.75915.a0. [DOI] [Google Scholar]
- 45.Kristensen R., Torp M., Kosiak B., Holst-Jensen A. Phylogeny and toxigenic potential is correlated in Fusarium species as revealed by partial translation elongation factor 1 alpha gene sequences. Mycol. Res. 2005;109:173–186. doi: 10.1017/S0953756204002114. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe M., Yonezawa T., Lee K.-I., Kumagai S., Sugita-Konishi Y., Goto K., Hara-Kudo Y. Molecular phylogeny of the higher and lower taxonomy of the Fusarium genus and differences in the evolutionary histories of multiple genes. BMC Evol. Biol. 2011;11:322. doi: 10.1186/1471-2148-11-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proctor R.H., Desjardins A.E., Plattner R.D., Hohn T.M. A polyketide synthase gene required for biosynthesis of fumonisin mycotoxins in Gibberella fujikuroi mating population. A. Fungal Genet. Biol. 1999;27:100–112. doi: 10.1006/fgbi.1999.1141. [DOI] [PubMed] [Google Scholar]
- 48.Glenn A.E., Zitomer N.C., Zimeri A.M., Williams L.D., Riley R.T., Proctor R.H. Transformation-mediated complementation of a FUM gene cluster deletion in Fusarium verticillioides restores both fumonisin production and pathogenicity on maize seedlings. Mol. Plant Microbe Interact. 2008;21:87–97. doi: 10.1094/MPMI-21-1-0087. [DOI] [PubMed] [Google Scholar]
- 49.Nelson P.E., Toussoun T.A., Marasas W.F.O. Fusarium Species. An Illustrated Manual for Identification. The Pennsylvania State University Press; White Oak, PA, USA: 1983. [Google Scholar]
- 50.Stępień Ł., Chełkowski J., Wenzel G., Mohler V. Combined use of linked markers for genotyping the Pm1 locus in common wheat. Cell. Mol. Biol. Lett. 2004;9:819–827. [PubMed] [Google Scholar]
- 51.Błaszczyk L., Tyrka M., Chełkowski J. PstIAFLP based markers for leaf rust resistance genes in common wheat. J. Appl. Genet. 2005;46:357–364. [PubMed] [Google Scholar]
- 52.Tamura K., Dudley J., Nei M., Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]