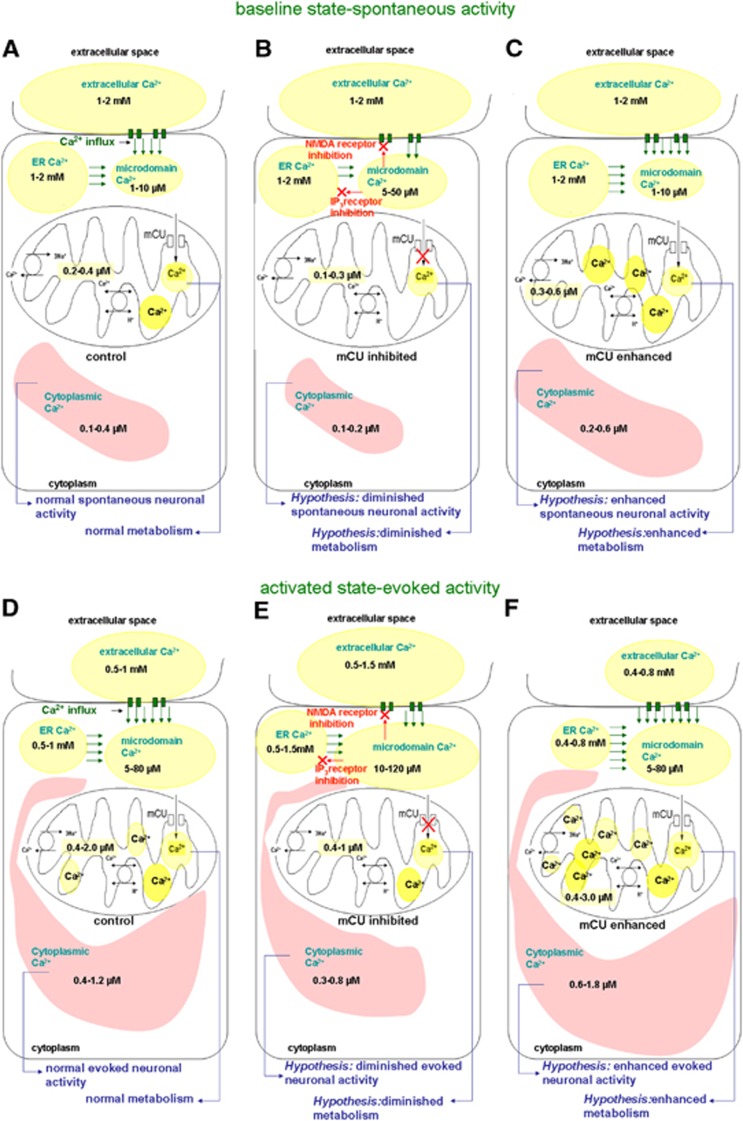
Figure 1.
Multicompartment distribution of free Ca2+ hypothesized in the brain in vivo during the baseline conditions (A–C) and evoked neural activity conditions (D–F) based on in vitro literature on Ca2+ measurements from neurons, glia, and brain slices.2, 4, 5, 7, 8, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 (D) During normal physiologic conditions, evoked brain activity can decrease extracellular and endoplasmic reticular Ca2+ ranges, and increase cytoplasmic and mitochondria Ca2+ ranges. (E) During mCU inhibition, evoked activity can lead to a relatively smaller decrease in extracellular and endoplasmic reticular (ER) Ca2+ ranges and smaller increase in cytoplasmic and mitochondrial Ca2+ ranges. Accumulating microdomain Ca2+ may inhibit plasma membrane glutamate receptors and mobilization from the ER stores resulting in reduced cytoplasmic Ca2+. This leads to the hypothesis that diminished mitochondrial Ca2+ influx capacity will reduce neocortical excitability, metabolism, and hemodynamic response. (F) During mCU enhancement, evoked activity can lead to relatively larger decreases in extracellular and ER Ca2+ ranges and relatively larger increases in cytoplasmic and mitochondrial Ca2+ ranges. Augmented clearance of Ca2+ in the microdomains by mitochondria may delay the Ca2+-mediated N-methyl-D-aspartate receptor desensitization resulting in larger cytoplasmic Ca2+ ranges. This leads to the hypothesis that enhanced mitochondrial Ca2+ influx capacity will increase neocortical excitability, metabolism, and hemodynamic response.