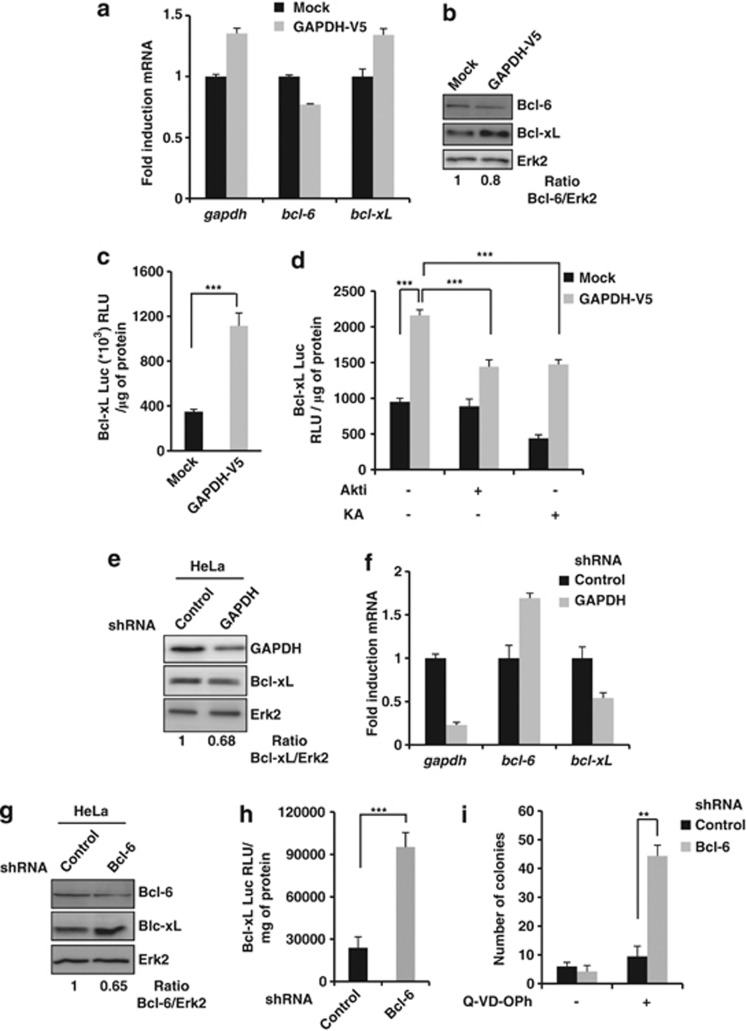
Figure 7.
GAPDH-dependent stabilization of active Akt leads to an increase in Bcl-xL expression via a decrease of Bcl-6. (a) Total mRNA was extracted from Mock and GAPDH-V5-overexpressing HeLa cells, and assessed by real-time RT-PCR for GAPDH, Bcl-6 and Bcl-xL expression. Relative mRNA values were determined, normalized to 18S and reported in terms of folds of the control. (b) As in panel a for assessing Bcl-6 and Bcl-xL protein level. The Bcl-6/Erk2 ratio was quantified and normalized to Mock cells. (c) Mock or GAPDH-overexpressing HeLa cells were transiently co-transfected with a Bcl-xL promoter–luciferase construct and a GFP-encoding vector. After 48 h, transfection efficiency and luciferase activity were assessed for each condition. (d) Cells were treated with or without 1 μM Akti or 0.5 μg/ml GAPDH inhibitor (KA) for 48 h and analyzed as described in panel c. (e and f) Proteins (e) or total mRNA (f) were extracted from HeLa cells transduced with either a control or an shRNA vector targeting GAPDH. GAPDH, Bcl-6 and Bcl-xL levels were assessed by western blot (e) or real-time RT-PCR (f). Relative mRNA values were determined, normalized to 18S and reported in terms of folds of the control. (g) Bcl-6 and Bcl-xL protein levels were assessed in HeLa cells transduced with either a control or an shRNA vector targeting Bcl-6. The Bcl-6/Erk2 ratio was quantified and normalized to Mock cells. (h) HeLa cells transduced with a control or a Bcl-6 shRNA vector were analyzed as described in panel c. (i) HeLa cells transduced as shown in h were analyzed as described in Figure 1d. Erk2 was used as a loading control. The data are the means of three independent experiments. ***P<0.005, **P<0.01, RLU, Relative Luciferase Unit