Abstract
Aims. The aim of this study is to compare our results of preoperative chemotherapy followed by pancreaticoduodenectomy (PD) with those of surgery alone in patients with localized resectable pancreatic ductal adenocarcinoma (PDAC). Methods. Outcome data for 112 patients of resectable PDAC who received preoperative chemoradiotherapy followed by PD (group I) between January 2004 and April 2010 were retrospectively analyzed and were compared with selected 120 patients who underwent PD alone (group II) in the same period. Results. Patients in group I had an incidence of locoregional recurrence of 17.1% compared to 30.8% in group II (P = 0.03). There were no statistically significant differences in postoperative morbidity (27.7% versus 30.8%) and mortality (2.67% versus 3.33%). The 1-, 2-, and 3-year survival rates were estimated at 82.1%, 54%, and 28%, respectively, with NCRT and 65.8%, 29.1%, and 10% without (P = 0.006). Nevertheless, preoperative chemotherapy did not reduce the 1-, 3-, and 5-year disease-free survival rates, which were estimated at 58%, 36.6%, and 12.5% with NCRT and 51.7%, 18.3%, and 7.5% without (P = 0.058). Conclusions. The treatment of NCRT followed by PD in patients with PDAC has a significantly lower rate of locoregional recurrence and a longer overall survival than those with surgery alone.
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a kind of remarkably highly lethal malignancy, foremost the 5th root cause of loss of life throughout the world [1]. Surgical resection has always been really the only most likely healing alternative. Even so, because of its ambitious tumor expansion as well as recurrence rate [2–4], in addition to the fact that a small section of patients are surgery candidates [5, 6], the actual survival rate of affected individuals is inadequate and simply ranges from 10% to 25.4% [7–10] at 5 years. The unsatisfying benefits of surgical treatment are only able to be enhanced by employing multidisciplinary treatments with adjuvant and neoadjuvant chemoradiotherapy (NCRT).
The additional current publications revealed survival advantages for PDAC patients with the use of adjuvant therapy postoperatively. A meta-analysis has been carried out by Stocken et al. [11] in 2005 from 5 randomized controlled trails, which revealed a 25% diminishment in risk of death in those who obtained chemotherapy and substantial 2 years of survival rates for those who received chemoradiotherapy, in contrast to those who did not (38% versus 25%). Alternatively, up to 30% of individuals had been incapable to complete the course of adjuvant treatment or to receive the designed amount of radiation or chemotherapy typically mainly because of the morbidity and continuous recuperation intervals following surgical treatment [12, 13].
In the contrast, the full course of prescribed chemotherapy is easily completed in NCRT without any delay, and it will presumably enhance effectiveness of chemoradiotherapy.
Even though an extreme variety of phase I/II studies [14, 15] have been published on the potential benefits for NCRT for patients with both resectable and unresectable PDAC, in addition to minimizing the possibilities of local tumor recurrence [16, 17], achieving better local tumor control [17, 18], or tumor downstaging with a subsequent potentially resectable tumor [19–21], unfortunately, no randomized controlled phase III trials comparing NCRT plus surgery versus surgical treatment only have been reported up till now, and as a consequence there are certainly no evidence-based medicine proofs that NCRT can offer any benefits for patients with PDAC. Within the distinction, the entire duration of prescribed chemoradiotherapy is definitely carried out with virtually no holdoff, and it can presumptively improve usefulness for PDAC patients.
Here we reported the principal experience with our large single institution by comparing 5-FU-based NCRT accompanied by PD with surgery alone. It is needed to be realized that NCRT is characterized as any preoperative chemoradiotherapy planning to increase the rate of microscopic tumor clearance and also to reduce the rate of tumor recurrence in this study.
2. Materials and Methods
2.1. Patients
Between January 2004 and April 2010, 232 consecutive patients with PDAC (limited to TI/T2 TNM staging) who were admitted to the Department of Hepatobiliary Pancreatic Surgery in our institution underwent PD, among whom 112 (48.7%) patients were treated with NCRT preoperatively, whereas the remaining 120 (51.3%) patients underwent PD alone. Patients were included in the study if they were pathologically proven to be PDAC cases postoperatively, and they were excluded if they were not amenable to operation, or if they were other cancer cases or with no cancers.
The approach to NCRT was determined and carried out by individual surgeons. The unique situation in our department was that 1 team of surgeons favored the use of NCRT and 1 team did not. Their choice of treatment was consistent over the period of the study, and this allowed for comparison of treatment between the 2 groups.
Preoperative evaluation of the staging of tumor consisted of physical examination, chest-radiography, abdomen contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic retrograde cholangiopancreatography (ERCP). All patients were required to meet the following eligible criteria for tumor resectability: tumors which do not involve major vascular structures including the celiac axis (CA), superior mesenteric artery (SMA), and superior mesenteric/portal vein complex and without extensive peripancreatic lymphadenopathy and/or the absence of distant metastases which were diagnosed radiologically before surgery.
After the completion of chemoradiotherapy, all patients were treated with pancreatic resection for curative intents and underwent no adjuvant chemoradiotherapy postoperatively. The median follow-up time for Group I was 28.6 months (range: 4–70 months) and 24.3 months (range: 9–67 months) for Group II.
2.2. Chemoradiotherapy
Chemotherapy was performed as neoadjuvant treatment in 81 of the 112 patients (96.4%). The main agents were 5-FU (600 mg/m2, d 1, 8, and 15 for 1 cycle) and gemcitabine (1000 mg/m2, d 1, 8, and 15 for 1 cycle). In the study that used only one regimen (n = 60), 38 (46.9%) patients were treated using 5-FU, and 35 (43.2%) patients used a gemcitabine-based regimen. Furthermore, gemcitabine and oxaliplatin combinations were used in 8 (9.8%) patients.
Thirty-one of the 112 patients (27.6%) received neoadjuvant radiotherapy (extrabody radiotherapy, EBRT). Doses applied ranged from 46 Gy/23 F to 50 Gy/23 F. No patients received both chemotherapy and radiotherapy.
2.3. Operative Finding
After four weeks of chemoradiotherapy, the planned pancreaticoduodenectomy (PD) or partial/total pancreatectomy were performed in all patients for curative intents. In our study, 124 patients (53.5%) underwent a classic PD (Whipple) and 76 (32.7%) underwent a pylorus-preserving PD (PPPD). Partial or total pancreatectomy was performed in 32 (14.8%) patients. R0 resection was achieved in 189 (81.4%) patients, of whom 92 patients were in the NCRT group and 97 patients were in the surgery-alone group. Pathologic specimens were reviewed and staged according to the American Joint Committee on Cancer (AJCC) Guidelines. Pathologic data regarding TNM staging, tumor size, histological differentiation grade, lymph node involvement, lymphovascular invasion, perineural invasion, and surgical margins were recorded.
2.4. Followup and Endpoints
All of the included patients were enrolled in our strict follow-up system. After discharge, serum CA-199 and an abdominal ultrasonography (US) and/or contrast-enhanced computed tomography scan was performed approximately 1 month for the initial three months after operation. Thereafter, we screened patients by tumor marker measurement and US every 3 months, and by helical CT every 6 months, and by ERCP or MRI when recurrence was suspected.
The endpoint of this study was time-to-recurrence which was defined as the period between initial pancreatectomy and the diagnosis of recurrence and time-to-death which calculated the duration from the date of transplantation to the date of death for any reasons. All followup data were summarized as of the end of August 2010.
2.5. Statistical Analysis
The Chi-square test or the Fisher exact test was used to evaluate the significant differences between the two groups. A proportion of patients with perioperative morbidity and mortality as well as tumor recurrence were compared between the two groups. The Kaplan-Meier curves were constructed for overall survival and disease-free survival, and the log-rank test was applied to compare the survival between the 2 groups of patients. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Comparison between the Two Groups
Patient demographics, including age, sex, body weight, height, and concurrent illness, were well matched in the two groups (Table 1). The size of the lesion, the histological differentiation, and the depth of tumor invasion in the two groups were also comparable (Table 2). Of the 232 patients, there were 144 (62.1%) males with a median age of 46.2 years (range: 17–67 years) and 88 (37.9%) females with a median age of 38.5 years (range: 24–54 years).
Table 1.
Clinical characteristics of the patients.
| Demographics | Group I | Group II | P values |
|---|---|---|---|
| Sex (male/female) | 75/37 | 69/51 | 0.773 |
| Age (year) | 45.9 ± 9.8 | 45.5 ± 9.3 | 0.672 |
| Heights (cm) | 166.5 ± 6.1 | 165.2 ± 5.8 | 0.914 |
| Body weight (kg/m2) | 57.6 ± 10.3 | 59.9 ± 7.6 | 0.254 |
| Concurrent illness | |||
| Hypertension | 21 | 24 | .ns* |
| Pulmonary tuberculosis | 4 | 3 | .ns |
| Diabetes mellitus | 13 | 10 | .ns |
| COPD* | 11 | 14 | .ns |
| Cholelithiasis | 16 | 22 | .ns |
| GERD* | 5 | 7 | .ns |
| Endometriosis | 4 | 4 | .ns |
| Others | 13 | 16 | .ns |
*COPD: chronic obstructive pulmonary diseases; *GERD: Gastroesophageal reflux disease; ns: not significant.
Table 2.
Operative and pathological characteristics of both groups.
| Characteristics | Group I | Group II | P values |
|---|---|---|---|
| Tumor location (head/body/tail) | 98/14 | 96/24 | .ns |
| Tumor size (mm) | 3.2 ± 1.3 | 3.5 ± 0.8 | .ns |
| Serum CA199 (U/mL) | 210.7 ± 45.6 | 284.3 ± 55.7 | .ns |
| Types of surgery | |||
| Whipple* | 65 | 59 | .ns |
| PPPD* | 35 | 41 | .ns |
| Partial or total pancreatectomy | 12 | 20 | .ns |
| Pathological differentiation (well/moderate/poor/others) | 12/74/23/3 | 14/76/25/5 | .ns |
| TNM* staging (I/II/III-IV) | 58/49/5 | 49/64/7 | .ns |
| Surgery margins (R0/R1/R2) | 95/17/0 | 96/24/0 | .ns |
| Operative time (min) | 615 ± 180 | 635 ± 210 | .ns |
| Blood loss (mL) | 1120 ± 350 | 1240 ± 430 | .ns |
| Hospital stay (day) | 11.5 ± 4.3 | 12.3 ± 3.5 | .ns |
*Whipple: standard pancreatoduodenectomy. PPPD: pylorus-preserving pancreatic resection. T: tumor. N: lymph nodes. M: metastasis.
3.2. Postoperative Morbidity and Mortality
Data regarding morbidity following neoadjuvant treatment and pancreatic resection were presented for 68 of 232 patients (Table 3). Morbidity included pancreatic fistula, which was defined as all suspect drainage with more than 300 IU/mL amylase-counting for more than 3 days; postoperative intraperitoneal hemorrhage (from arterial or venous vessel, operative field, and gastrointestinal track); lymphorrhea (colorless drainage of more than 300 mL for more than 10 postoperative days); diarrhea (more than three liquid exonerations per day for more than 10 days); delayed gastric emptying, which was calculated by the nasogastric tube (NGT) left in place for 3 days or reinsered because of repeated emesis after removal of the NGT being unable to tolerate a solid diet after the 7th postoperative day; abdominal infection (after the 3rd postoperative day, fever, abdominal distension, and intestinal paralysis appear and last for 24–48 hours, with leukocytosis, hypoproteinemia, and anemia; fluid accumulation is found radiologically); small bowel infraction; pulmonary embolization; atelectasis; and wound infections (Table 4).
Table 3.
Postoperative mortality and morbidity between the two groups.
| Objects | All patients | Group I | Group II | P values |
|---|---|---|---|---|
| Morbidity | 29.3% | 27.7% | 30.8% | 0.123 |
| Mortality | 3.02% | 2.67% | 3.33% | 0.123 |
Table 4.
Number of complications between the two groups.
| Complications | Group I | Group II |
|---|---|---|
| Pancreatic fistula | 7 | 9 |
| Intraperitoneal hemorrhage | 3 | 4 |
| Lymphorrhea | 5 | 6 |
| Small bowel infarction | 2 | 2 |
| Diarrhea | 4 | 5 |
| Pulmonary embolization | 2 | 1 |
| Atelectasis | 3 | 2 |
| Delayed gastric emptying | 5 | 8 |
|
| ||
| Total | 31 | 37 |
The postoperative mortality was calculated as death from any causes within 45 days postoperatively. Postoperative mortality was 2.67% for patients in Group I and 3.33% in Group II. Mortality was not statistically different in the two groups.
3.3. Overall Survivals (OS)
The analysis of the OS curves between the two groups was revealed in Figure 1, which demonstrated that there was a statistically significant difference between the two groups (P = 0.006). The overall survival rates for the 112 patients in NCRT group at 1, 3, and 5 years were 76%, 55%, and 22%, respectively, whereas they were 44%, 25%, and 9% in the surgery-alone group, respectively.
Figure 1.
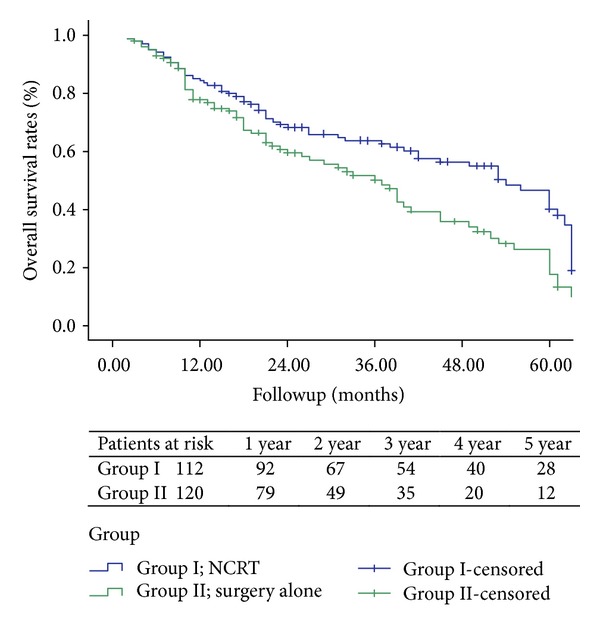
Showing the Kaplan-Meier overall survival curve for the 112 patients receiving NCRT and the 120 patients receiving surgery resection alone. There was no significant difference in overall survival between the two groups (P = 0.006).
3.4. Disease-Free Survivals (DFS)
The Kaplan-Meier DFS curves of patients between the two groups were compared in Figure 2, which revealed that the DFS was longer in the NCRT group, with the disease-free survival rate of 58% at 1 year, 36.6% at 3 years, and 12.5% at 5 years, and it was 51.7% at 1 year, 22% at 3 years, and 7.5% at 5 years in the surgery-alone group. However, the DFS was not significantly different when the two groups were compared (P = 0.058).
Figure 2.
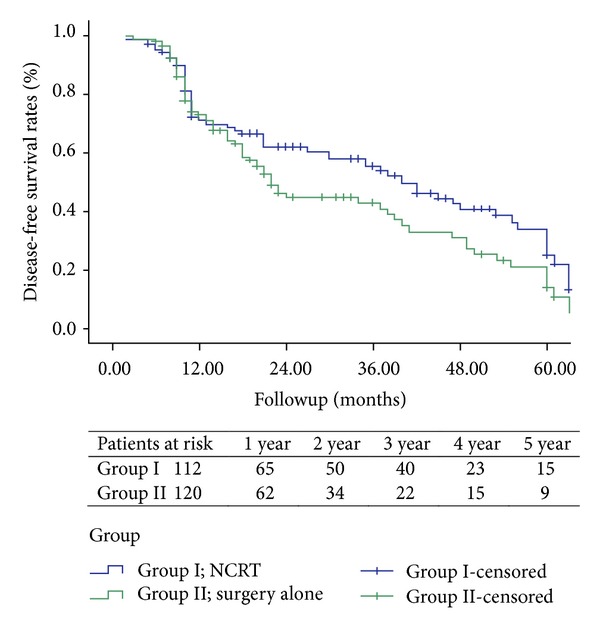
Showing the Kaplan-Meier disease-free survival curve for patients undergoing NCRT (n = 112) compared with those patients undergoing surgery resection alone (n = 120). There was no statistical difference between the two groups (P = 0.058).
3.5. Tumor Recurrences
Tumor recurrences were observed in 176 patients. The recurrence rates were 35.3% for the NCRT group and 40.5% for the surgery-alone group, respectively. Focusing on the clinical pathological features of all patients, there were no significant differences between the two groups (P < 0.05). Intrahepatic and locoregional lymph nodes metastases were the main first or primary locations of cancer recurrence in both groups (Table 5). Although the overall tumor recurrence rates were not statistically different between the two groups, patients receiving NCRT were more likely to have lower frequency of local lymph node metastasis than patients receiving surgery alone. The frequencies of other locations of postoperative tumor recurrence were similar between the two groups (4.9% versus 5.3%; P = 0.02).
Table 5.
Comparison between the two groups regarding the first location of tumor recurrences.
| Metastasis site | Group I (no. and %) | Group II (no. and %) | P values |
|---|---|---|---|
| Intrahepatic | 35 (42.7%) | 31 (33.1%) | .ns |
| Locoregional | 14 (17.1%) | 29 (30.8%) | 0.032 |
| Peritoneal | 12 (14.8%) | 11 (11.7%) | .ns |
| Pulmonary | 10 (12.2%) | 12 (12.9%) | .ns |
| Retroperitoneal | 7 (8.5%) | 6 (6.4%) | .ns |
| Others | 4 (4.9%) | 5 (5.3%) | .ns |
4. Discussion
Preoperative chemoradiotherapy is usually a neoadjuvant treatment method. Even though the effective use of adjuvant chemotherapy and radiotherapy in addition to intraoperative radiotherapy (IORT) or EBRT (extrabody radiotherapy) can partially control the local tumor growth and reduce tumor recurrence, there seemed to be tiny impact on the increase of the rate of survival of patients who underwent such procedure, so neoadjuvant therapies are actually suggested by some surgeons, aiming to enhance the resection rate and the 5-year survivals. Theoretically, preoperative chemoradiotherapy has the following advantages when compared with the adjuvant therapy in patients with pancreatic cancer. (1) It can complete the path of adjuvant treatment or obtain the organized quantity of chemotherapy or radiation with no delay virtually [22]; (2) it can downstage tumor classification enabling an improved tumor oncological clearance along with a higher negative surgical margin (R0 resection) [23, 24]; (3) it limits the possible likelihood of cancerous growth seeding due to intraoperative manipulation [25]; (4) it is much more prone to endure it (chemoradiotherapy) prior to surgery; (5) it blocks the oxygen supply to the tumor cells and kills them effectively; and (6) it minimizes the potential risk of pancreatic anastomotic leakage.
NCRT may additionally improve survival after resection for patients with PDAC. Nonetheless, there is actually constrained information concerning the role of NCRT for pancreatic cancer in clinical practice. Preoperative chemotherapy and radiotherapy are nevertheless a place of disputes. Pendurthi et al. [26] retrospectively abbreviated the information of 70 patients who received preoperative and postoperative chemoradiotherapy and found that 27 patients who underwent preoperative chemoradiotherapy were more unlikely to possess lymph node involvement (28% versus 87%, P < 0.0006) and a lower rate of positive surgery margins (28% versus 56%, P = ns) compared with the 43 patients who obtained chemoradiotherapy after surgery, but there were no significant differences between the two groups in overall survival rates and local tumor control. Similarly, Evans and Pisters [27] from the Department of Anderson Cancer Center confirmed that preoperative chemoradiotherapy did not increase postoperative morbidity, the 3-year survival rate reached 23%, and a less probability of local tumor recurrence was witnessed as a result of a long-term follow-up. In addition, in the Stanford Cancer Center, Joseph Cetal [28] found that preoperative chemoradiotherapy was tolerated in locally advanced tumors without having to incorporate the operation risk and downstage the tumor TNM stage, as well as to increase the oncological clearance rate.
The role of preoperative chemoradiotherapy in extended survival for patients with pancreatic cancer remains not noticeable currently, but NCRT has been considered as one of the most reliable treatment methods within the treatments for individuals with locally advanced pancreatic cancer.
The parameters used to classify the pancreatic cancer into resectable category are typically depending on primary tumor TNM stage, lymph nodes status, and adjacent major organs conditions. The surgical resection margin status and the existence of lymph nodes metastases were found to be the most significant determinants of survival after surgery [29, 30]. In our study, patients who received pancreatectomy alone were more prone to have local lymph node metastasis (P < 0.001). Overall survival was statistically different for those who received neoadjuvant therapy when compared with those who received surgery alone (P = 0.969), even though there was no statistical difference in disease-free survivals among the two groups.
Our study can be criticized for its patient selection and lack of pathological diagnosis preoperatively, as well as the retrospective nature of assessment of outcome. Nonetheless, considering the existing debate in the application of NCRT for PDAC, it highlights several significant things for surgeons. First, it is very important to determine which patients are more or less likely to benefit from the use of NCRT. Second, the preoperative criteria and definitions for resectability and unresectability are clearly keys and have to be standardized, and the necessity for a histological diagnosis is additionally an essential point for the patients who are initially unresectable. Third, it is also considerable to distinguish between the prognostically favorable groups of patients with intrapancreatic bile duct cancer from individuals with PADC due to the effects of the neoadjuvant treatment on histology of the primary tumor. Additionally, the survival rates reported in our research also compare and contrast positively towards the survival data of other retrospective neoadjuvant treatment series.
5. Conclusion
The frequently acknowledged conventional strategy to affected individuals with resectable pancreatic tumors is pancreaticoduodenectomy accompanied by 5-FU-based chemotherapy or radiotherapy. In the absence of randomized controlled trails, the application of neoadjuvant therapy for resectable pancreatic cancer remains disputable. For marginally unresectable tumors, neoadjuvant chemoradiotherapy may turn out to be a highly effective strategy for determining which patients might possibly benefit from surgical exploration and experimented with resection.
Authors' Contribution
Hui Jiang and Chi Du contributed equally to the study.
References
- 1.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1996. Ca-A Cancer Journal for Clinicians. 1996;46(1):5–27. doi: 10.3322/canjclin.46.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Manabe T, Ohshio G, Baba N, et al. Radical pancreatectomy for ductal cell carcinoma of the head of the pancreas. Cancer. 1989;64(5):1132–1137. doi: 10.1002/1097-0142(19890901)64:5<1132::aid-cncr2820640528>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Baumel H, Huguier M, Manderscheid JC, Fabre JM, Houry S, Fagot H. Results of resection for cancer of the exocrine pancreas: a study from the French Association of Surgery. British Journal of Surgery. 1994;81(1):102–107. doi: 10.1002/bjs.1800810138. [DOI] [PubMed] [Google Scholar]
- 4.Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. doi: 10.1002/1097-0142(19900701)66:1<56::aid-cncr2820660112>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Mehta VK, Fisher G, Ford JA, et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. Journal of Gastrointestinal Surgery. 2001;5(1):27–35. doi: 10.1016/s1091-255x(01)80010-x. [DOI] [PubMed] [Google Scholar]
- 6.Ghaneh P, Sultana A, Shore S, Stocken D, Neoptolemos J. The case for adjuvant chemotherapy in pancreatic cancer. Best Practice and Research. 2006;20(2):383–401. doi: 10.1016/j.bpg.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Annals of Surgery. 2006;244(1):10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-Year experience. World Journal of Surgery. 2003;27(3):324–329. doi: 10.1007/s00268-002-6659-z. [DOI] [PubMed] [Google Scholar]
- 9.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Büchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. British Journal of Surgery. 2004;91(5):586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 10.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma: clinicopathologic analysis of 5-year survivors. Annals of Surgery. 1996;223(3):273–279. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stocken DD, Büchler MW, Dervenis C, et al. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. British Journal of Cancer. 2005;92(8):1372–1381. doi: 10.1038/sj.bjc.6602513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. Journal of Clinical Oncology. 1997;15(3):928–937. doi: 10.1200/JCO.1997.15.3.928. [DOI] [PubMed] [Google Scholar]
- 13.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Annals of Surgery. 1999;230(6):776–784. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turrini O, Viret F, Moureau-Zabotto L, et al. Neoadjuvant chemoradiation and pancreaticoduodenectomy for initially locally advanced head pancreatic adenocarcinoma. European Journal of Surgical Oncology. 2009;35(12):1306–1311. doi: 10.1016/j.ejso.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Turrini O, Ychou M, Moureau-Zabotto L, et al. Neoadjuvant docetaxel-based chemoradiation for resectable adenocarcinoma of the pancreas: new neoadjuvant regimen was safe and provided an interesting pathologic response. European Journal of Surgical Oncology. 2010;36(10):987–992. doi: 10.1016/j.ejso.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Stessin AM, Meyer JE, Sherr DL. Neoadjuvant radiation is associated with improved survival in patients with resectable pancreatic cancer: an analysis of data from the surveillance, epidemiology, and end results (SEER) registry. International Journal of Radiation Oncology Biology Physics. 2008;72(4):1128–1133. doi: 10.1016/j.ijrobp.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 17.Raut CP, Evans DB, Crane CH, Pisters PWT, Wolff RA. Neoadjuvant therapy for resectable pancreatic cancer. Surgical Oncology Clinics of North America. 2004;13(4):639–661. doi: 10.1016/j.soc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 18.White RR, Tyler DS. Neoadjuvant therapy for pancreatic cancer: the Duke experience. Surgical Oncology Clinics of North America. 2004;13(4):675–684. doi: 10.1016/j.soc.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Czischke K, Brennan MF, Conlon KC. Does neoadjuvant chemoradiation downstage locally advanced pancreatic cancer? Journal of Gastrointestinal Surgery. 2002;6(5):763–769. doi: 10.1016/s1091-255x(02)00017-3. [DOI] [PubMed] [Google Scholar]
- 20.Moutardier V, Turrini O, Huiart L, et al. A reappraisal of preoperative chemoradiation for localized pancreatic head ductal adenocarcinoma in a 5-year single-institution experience. Journal of Gastrointestinal Surgery. 2004;8(4):502–510. doi: 10.1016/j.gassur.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Brown KM, Siripurapu V, Davidson M, et al. Chemoradiation followed by chemotherapy before resection for borderline pancreatic adenocarcinoma. American Journal of Surgery. 2008;195(3):318–321. doi: 10.1016/j.amjsurg.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Tse RV, Dawson LA, Wei A, Moore M. Neoadjuvant treatment for pancreatic cancer-A review. Critical Reviews in Oncology/Hematology. 2008;65(3):263–274. doi: 10.1016/j.critrevonc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Cleary SP, Gryfe R, Guindi M, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. Journal of the American College of Surgeons. 2004;198(5):722–731. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Pingpank JF, Hoffman JP, Ross EA, et al. Effect of preoperative chemoradiotherapy on surgical margin status of resected adenocarcinoma of the head of the pancreas. Journal of Gastrointestinal Surgery. 2001;5(2):121–130. doi: 10.1016/s1091-255x(01)80023-8. [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa O, Ohigashi H, Imaoka S, et al. Is the long-term survival rate improved by preoperative irradiation prior to Whipple’s procedure for adenocarcinoma of the pancreatic head? Archives of Surgery. 1994;129(10):1075–1080. doi: 10.1001/archsurg.1994.01420340089017. [DOI] [PubMed] [Google Scholar]
- 26.Pendurthi TK, Hoffman JP, Ross E, Johnson DE, Eisenberg BL. Preoperative versus postoperative chemoradiation for patients with resected pancreatic adenocarcinoma. American Surgeon. 1998;64(7):686–692. [PubMed] [Google Scholar]
- 27.Evans DB, Pisters PWT. Commentary: preoperative chemoradiation therapy for pancreatic cancer. Surgical Clinics of North America. 2001;81(3):709–713. doi: 10.1016/s0039-6109(05)70156-0. [DOI] [PubMed] [Google Scholar]
- 28.Poen JC, Ford JM, Niederhuber JE. Chemoradiotherapy in the management of localized tumors of the pancreas. Annals of Surgical Oncology. 1999;6(1):117–122. doi: 10.1007/s10434-999-0117-1. [DOI] [PubMed] [Google Scholar]
- 29.Hein Allema J, Reinders ME, van Gulik TM, Koelemay MJW, van Leeuwen DJ, de Wit LTh, et al. Prognostic factors for survival after pancreaticoduodenectomy for patients with carcinoma of the pancreatic head region. Cancer. 1995;75:2069–2076. doi: 10.1002/1097-0142(19950415)75:8<2069::aid-cncr2820750807>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Annals of Surgery. 2003;237(1):74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]


