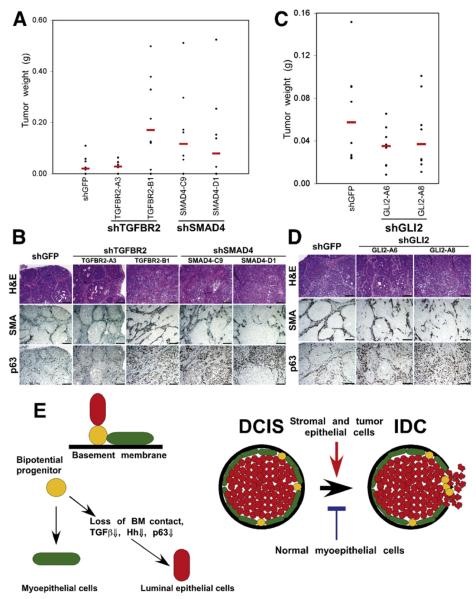
Figure 5. Pathways Regulating In Situ to Invasive Carcinoma Progression.
(A and B) The effect of loss of TGFβ signaling in MCFDCIS cells on tumor weight (A) and histology (B). Downregulation of TGFBR2 (in one of the clones) or SMAD4 increased tumor weight (TGFBR2-A3, p = 0.7899; TGFBR2-B1, p = 0.02996; SMAD4-C9, p = 0.09847; SMAD4-D1, p = 0.2379) and resulted in invasive tumors.
(C and D) The effect of decreased Gli2 expression in MCFDCIS cells on tumor weight (C) and histology (D). Downregulation of Gli2 decreased tumor weight (GLI2-A6, p = 0.1605; GLI2-A8, p = 0.2786), but increased invasive histology. Scale bars correspond to 100 μm in all panels.
(E) Hypothetical model summarizing our results and explaining in situ to invasive breast carcinoma progression. Bipotential mammary epithelial progenitor cells can give rise to myoepithelial and luminal cells. Detachment from the basement membrane (BM) and subsequent downregulation of TGFβ, Hh, integrin-ECM, and p63 pathways lead to luminal epithelial differentiation. Myoepithelial cells are necessary for the formation of DCIS. Stromal fibroblasts promote while normal myoepithelial cells inhibit tumor progression in part through their effects on the BM.