Abstract
Advanced urothelial cancer is associated with a poor prognosis and there has been no substantial progress over the past three decades since the development of platinum-based multiagent chemotherapy. Clinical trials evaluating novel agents and combinations including chemotherapeutic drugs, as well as targeted inhibitors, are desperately needed. With a better understanding of the complex molecular alterations that drive urothelial tumorigenesis, new targets for novel therapeutics are being defined. This article will describe the current state of advanced urothelial cancer treatment and provide a comprehensive discussion of novel agents in development.
Keywords: bladder cancer, targeted therapy, transitional cell carcinoma, urothelial cancer
In the USA, bladder cancer is the fourth most common malignancy in men and the eighth most common in women [1]. Approximately 70,980 new cases of bladder cancer were predicted for the year 2009 with an estimated 14,330 deaths [1]. In total, 75% of patients present with non-muscle-invasive (Ta, T1 and Tis) disease, which is treated with a transurethral resection of the bladder tumor (TURBT) with or without intravesical therapy [2,3]. An estimated 70% of these individuals will experience a recurrence and 10–20% of these recurrences will progress to muscle-invasive disease [4]. A minority of patients is diagnosed with either muscle-invasive (20%) or metastatic (5%) disease at the time of presentation. Patients with muscle-invasive disease who are surgical candidates generally undergo a radical cystectomy with pelvic lymph node dissection, with only a minority of these patients receiving perioperative chemotherapy. The mainstay of treatment for those individuals with metastatic disease is systemic chemotherapy alone [5]. In the first-line, meta-static setting, treatment with cisplatin-based chemotherapy results in a modest improvement in survival, with a median survival of approximately 12–15 months [6,7]. Unfortunately, no US FDA-approved therapy exists in the second-line setting, that is, for patients who progress after first-line platinum-based chemotherapy. For these reasons, urothelial cancer (UC) is a devastating illness with ongoing research aimed at developing active therapies including novel targeted agents in patients with advanced disease. This article focuses on the current management strategy for patients with relapsed or refractory advanced UC, as well as novel therapies in development including targeted agents.
Standard chemotherapy regimens
Cisplatin-based combination chemotherapy represents the standard of care for first-line treatment of advanced UC. Early trials examined the efficacy of cisplatin administered with methotrexate [8]. Later studies found that the three-drug combination including cisplatin, methotrexate and vinblastine resulted in a response rate (RR) of 56% and a median overall survival (OS) of 8 months [9]. A regimen consisting of methotrexate, vinblastine, doxorubicin and cisplatin (M-VAC) was initially tested in 25 patients with metastatic UC with a 50% rate of complete remission and a 13-month median survival [6]. Based on these promising results, M-VAC was subsequently compared with cisplatin alone in a prospective, randomized trial and was found to be superior with respect to overall RR (ORR; 39 vs 12%; p < 0.0001), progression-free survival (PFS; 10 vs 4.3 months) and OS (12.5 vs 8.2 months) [10]. A 6-year follow-up confirmed superior survival in those patients who received M-VAC, although only 3.7% of these patients were still alive and without evidence of recurrent disease, a finding that underscores the limited durability of response to systemic chemotherapy in advanced bladder cancer [11]. Of note, prognostic factors predicting survival have been derived from five clinical trials examining first-line M-VAC therapy in patients with metastatic or unresectable UC, and have been utilized to stratify patients into risk groups based upon the probability of disease-specific mortality. These factors include Karnofsky performance status (KPS) below 80% and the presence of visceral metastases (lung, liver or bone) [12]. Patients with zero, one or two risk factors have median survival times of 33, 13.4 and 9.3 months, respectively [12].
Gemcitabine and docetaxel were determined to be active single agents in bladder cancer and were subsequently evaluated in combination with cisplatin [13–15]. While the combination of docetaxel and cisplatin was found to be inferior to M-VAC, a Phase III trial comparing gemcitabine plus cisplatin (GC) and M-VAC in locally advanced or metastatic disease revealed similar RRs (46% with GC vs 49% with M-VAC), time to progression (7.4 months in both arms) and median survival (13.8 vs 14.8 months) [7,16]. Patients randomized to M-VAC experienced significantly higher rates of grade 3–4 neutropenia with subsequent complications of fever and sepsis [7]. Based on these data, GC has emerged as a standard of care for the first-line treatment of patients with metastatic UC.
Several triplet chemotherapy regimens have been investigated in Phase II clinical trials including agents such as ifosfamide and paclitaxel [17]. A Phase I/II study evaluated the combination of paclitaxel, cisplatin and gemcitabine (PCG) in 61 patients; the ORR was 78% with Phase I and II median survivals of 24 and 16 months, respectively [18]. A multicenter Phase III study coordinated by the European Organisation for Research and Treatment of Cancer randomized 627 patients with metastatic bladder cancer to receive GC or PCG [19–24]. While the PCG arm demonstrated a superior ORR (57% with PCG vs 46% with GC; p = 0.02), no significant difference was noted in either PFS or OS, although an unplanned subgroup analysis revealed that patients with a bladder primary tumor had an improvement in OS (p = 0.03) [24]. This benefit in OS was only observed in the previously described risk groups 0 or 1. Thrombocytopenia and bleeding were seen more often with GC (12 vs 7%) while febrile neutropenia was more prevalent with PCG (13 vs 4%).
In general, Phase II trials of second-line therapy for metastatic disease have resulted in limited activity with responses seen in 10–20% of patients and a PFS of 2–3 months with a median survival of approximately 6 months [25]. Uni- and multivariate analyses of 370 patients with metastatic platinum-refractory UC identified the presence of liver metastases, a European Cooperative Oncology Group (ECOG) performance status (PS) greater than 0, and hemoglobin value less than 10 g/dl as pre treatment prognostic factors for patients failing platinum-containing regimens [26]. The median OS for patients with zero, one, two or three factors was 14.2, 7.3, 3.8 and 1.7 months (p < 0.001), respectively [26]. Although no FDA-approved standard of care exists for the treatment of advanced UC in the second-line setting, the current National Comprehensive Cancer Network (NCCN) guidelines suggest the use of a number of chemotherapeutic agents, including a taxane or pemetrexed, while strongly encouraging participation in a clinical trial.
While prognostic markers for survival have been established for advanced UC, biomarkers predicting response to therapy are lacking. Such biomarkers would be especially useful in predicting resistance to platinum agents since cisplatin represents the cornerstone of traditional chemotherapy in UC, so that patients predicted to possess platinum resistance can preferentially be enrolled in clinical trials investigating alternative agents. In one retrospective analysis, mRNA-expression profiling of pretreatment tumors from 57 patients who had received GC or GC plus paclitaxel as part of two clinical trials identified a correlation between expression of the excision repair cross-complementation group 1 (ERCC1) gene and OS [27]. Patients with low ERCC1 mRNA levels were found to have a significantly increased OS compared with patients with high transcript levels (25.5 vs 15.4 months; p = 0.03). ERCC1 levels did not correlate with response to chemotherapy; however, since ERCC1 is an integral component of the cellular nucleotide excision repair machinery that removes bulky platinum-induced DNA adducts, high ERCC1 levels may represent heightened DNA repair capability and, therefore, a reduced response to cisplatin. Other molecular mechanisms have been identified that lead to platinum resistance, including reduced intracellular accumulation of drug owing to either increased drug efflux or trapping within intracellular compartments such as endosomes, drug inactivation and the ability to escape apoptosis following DNA damage. The identification of gene signatures predictive of cisplatin resistance is ongoing. A potent method to construct such gene-expression models in bladder cancer involves the identification of candidate biomarker genes that predict response to a compound of interest and that are differentially expressed in resistant and sensitive cell lines within the NCI-60 reference panel of 60 representative cancer cell lines [28]. The candidate genes are then filtered through a co-expression extrapolation (COXEN) algorithm to isolate those genes that are expressed both within the NCI-60 reference panel and within a panel of bladder cancer cell lines following exposure to the compound of interest. These filtered genes are potentially the most relevant for prediction of sensitivity to a given compound and can be utilized to construct a gene-expression signature for that compound. Such gene-expression signatures can be used to design therapeutic clinical trials and to inform clinicians prospectively about the potential utility of specific chemotherapeutic agents for individual patients.
Novel chemotherapy agents
Table 1 summarizes select novel chemotherapy agents in development for advanced UC.
Table 1.
Novel chemotherapeutic agents in advanced urothelial Cancer.
| Agent | Phase/n | RR (%) | OS (months) | Ref. |
|---|---|---|---|---|
| Ixabepilone | II/45 | 12 | 8 | [31] |
|
| ||||
| Pemetrexed | II/47 | 27.7 | 9.6 | [33] |
| II/13 | 8 | NA | [34] | |
|
| ||||
| Pralatrexate | II/41 | Ongoing | Ongoing | |
|
| ||||
| Vinflunine | II/57 | 18 | 6.6 | [36] |
| II/151 | 15 | 8.2 | [37] | |
| III/370 | 8.6 | 6.9 | [38] | |
|
| ||||
| Larotaxel | III/900 | Closed to accrual | Closed to accrual | |
|
| ||||
| Tesetaxel | Planned | |||
|
| ||||
| Albumin-bound paclitaxel | II/48 | Ongoing | Ongoing | [43] |
|
| ||||
| Eribulin | II/40 | 38 | 9.4 | [47] |
NA: Not applicable; OS: Overall survival; RR: Response rate.
Epothilones
The epothilones are a class of chemotherapeutic agents that stabilize microtubule polymers leading to mitotic arrest and inhibition of cell proliferation [29]. Preclinical data suggested antineoplastic activity for ixabepilone, a semisynthetic analog of the natural myxobacterial product epothilone B, in human cancer xenografts, including paclitaxel-resistant tumors [30]. Ixabepilone was subsequently FDA approved for the treatment of progressive, metastatic or locally advanced breast cancer. Based upon these findings, a Phase II trial was conducted in which 45 previously treated patients with recurrent UC were administered ixabepilone 40 mg/m2 every 21 days until disease progression or toxicity [31]. In total, 17 patients (40%) had received prior taxane therapy. The ORR was 11.9%, with five patients achieving an objective partial response (PR). The median PFS was 2.7 months (95% CI: 1.8–4.1 months) with an OS of 8 months (95% CI: 4.9–9.8 months). Moderate toxicity was observed including 12 patients (26%) with grade 4 toxicities. In total, 16 patients experienced grade 3 or 4 granulocytopenia with one treatment-related death related to febrile neutropenia. Three patients developed grade 3 or 4 sensory neuropathy and one patient developed grade 3 renal failure [31]. Based on the toxicity profile and only modest activity, the investigators decided against further investigation of ixabepilone in advanced UC [31].
Pemetrexed
Pemetrexed, a multi-targeted antifolate analog that inhibits the synthesis of nucleotide precursors, is FDA approved for the treatment of both non-small-cell lung cancer (NSCLC) and malignant mesothelioma, and has also been evaluated in the second-line setting in UC. An initial trial by Paz-Ares et al. investigated pemetrexed as salvage chemotherapy in UC [32]. Owing to significant toxicity in the first six enrolled patients, including two toxic deaths from sepsis and renal failure, the trial required dose reduction from 600 to 500 mg/m2. The ORR was 29% (95% CI: 14–48%). In a Phase II study led by the Hoosier Oncology Group (IN, USA), 47 patients received pemetrexed 500 mg/m2 every 3 weeks with vitamin B12 and folic acid supplementation [33]. This trial resulted in an ORR of 27.7%, including three complete responses (CRs). Median time to disease progression was 2.9 months (95% CI: 1.7–4.6 months) with a median OS of 9.6 months (95% CI: 5.1–13.8 months). In a study performed at the Memorial Sloan–Kettering Cancer Center (MSKCC; NY, USA), 13 patients with relapsed disease received second-line therapy with pemetrexed at 500 mg/m2 every 21 days, with an objective response seen in only one patient with an ORR of 8% (95% CI: 0–29%) [34]. Pemetrexed is well tolerated and represents an option for the treatment of patients with advanced UC in the second-line setting.
Pralatrexate
Pralatrexate is a novel targeted antifolate that has been FDA approved for the treatment of relapsed or refractory peripheral T-cell lymphoma, and early-phase clinical trials also suggest efficacy and tolerability in NSCLC. The drug binds to the reduced folate carrier (RFC)-1 cell surface receptor, which is overexpressed on cancer cells, and its structure allows for increased intracellular retention once it has been taken up by the target cell. A single-arm Phase II trial is investigating the efficacy of pralatrexate in patients with metastatic UC that has progressed on platinum-based therapy. The primary end point is objective RR and secondary end points include PFS, OS and tolerability. The results of this trial are pending, although pralatrexate was recently provided an orphan drug designation by the FDA for treatment of bladder cancer.
Vinflunine
Vinflunine is a novel fluorinated vinca alkaloid that inhibits the assembly of tubulin monomers, resulting in mitotic arrest and subsequent apoptosis. It has a favorable neurotoxicity profile compared with other vinca alkaloids as a result of reduced binding affinity for tubulin and is also effective in multiple solid tumors, including breast and NSCLC [35]. Based on preclinical activity against the murine bladder cancer cell line MB-49, Vinflunine was first investigated in 57 patients with recurrent disease or progression of disease within 1 year of chemotherapy [36]. The drug was initially dosed at 350 mg/m2 in six patients; however, a program-wide safety evaluation performed in all Phase II trials of Vinflunine led to a dose reduction to 320 mg/m2. All six patients treated for a total of 15 cycles at 350 mg/m2 experienced myelosuppression, with one death related to febrile neutropenia. Nine out of 51 patients who received Vinflunine at 320 mg/m2 achieved a PR with an ORR of 18% (95% CI: 8.4–30.9%). Of note, five of the nine PRs occurred at visceral sites of metastases, including the lung and liver. In the patients who experienced a PR, eight out of 34 responses occurred in individuals treated for metastatic disease in the first-line setting. The median PFS was 3 months (95% CI: 2.4–3.8 months), with a median OS of 6.6 months (95% CI: 4.8–7.6 months) and the median response duration was 9.1 months (95% CI: 4.2–15 months). A total of 34 patients (67%) experienced grade 3 or 4 neutropenia, with five (10%) cases of febrile neutropenia. Grade 3 or 4 constipation was observed in four patients (8%). A second Phase II trial investigated Vinflunine in 151 patients with platinum-refractory UC [37]. Patients who were 75 years of age or older, with a history of pelvic irradiation, a KPS of 80–90% or with a creatinine clearance of 60 ml/min or less were administered 280 mg/m2. All other patients received 320 mg/m2. The ORR was 14.9%, with a median duration of response of 6.8 months in an interim analysis of 114 patients. Grade 3 or 4 neutropenia, anemia and thrombocytopenia occurred in 59, 17 and 4% of patients, respectively. Grade 3 or 4 constipation was observed in 14% of patients. Given the activity seen in the Phase II studies, a Phase III trial was performed and reported by Bellmunt et al. in which 370 patients with previously treated advanced UC were randomized to vinflunine at a dose of 320 mg/m2 every 3 weeks with best supportive care (BSC) or to BSC alone [38]. Those patients with an ECOG PS of 1 or an ECOG PS of 0 but with a history of prior pelvic irradiation received a dose of 280 mg/m2 with the initial cycle followed by dose escalation to 320 mg/m2 in the absence of significant hematologic toxicity. Survival was improved in the Vinflunine arm at 6.9 months compared with 4.6 months with BSC alone, although this was not a significant difference in the intent to treat population with a hazard ratio of 0.88 (95% CI: 0.69–1.12; p = 0.287). A multivariate Cox analysis adjusting for prognostic factors revealed a statistically significant effect of Vinflunine on OS (p = 0.036) with a hazard ratio of 0.77 (95% CI: 0.61–0.98). In the eligible population (n = 357), the median OS was significantly longer for the Vinflunine arm compared with BSC (6.9 vs 4.3 months; p = 0.040). On the Vinflunine arm, toxicities including grade 3 or 4 neutropenia and neutropenic fever occurred in 50 and 6% of patients, respectively. Grade 3 or 4 constipation was observed in 16% of patients, mainly during the initial two cycles of therapy. A low incidence of peripheral neuropathy was noted. There are no ongoing UC clinical trials with Vinflunine.
Larotaxel
The novel taxane larotaxel stabilizes tubulin polymers, preventing disassembly of the microtubules and thereby inhibiting cell division. Preclinical studies in melanoma and breast cancer cell lines suggested improved efficacy over docetaxel in taxane-resistant cancer models. Early-phase clinical trials defined dose-limiting toxicities as grade 3–4 neutropenia and diarrhea. Phase II studies revealed clinical efficacy for larotaxel in taxane-resistant meta-static breast cancer, as well as in the first-line setting in combination with platinum in stage III or IV NSCLC [39]. A Phase III trial was designed in the setting of locally advanced or metastatic UC in which patients were randomized to receive front-line cisplatin plus larotaxel or gemcitabine plus cisplatin; this trial has since been closed to accrual.
Tesetaxel
Another microtubule-stabilizing taxane in early-phase clinical trials is the oral agent tesetaxel. This drug has undergone Phase II testing in metastatic platinum-refractory NSCLC, fluorouracil-refractory advanced gastric cancer and metastatic colon cancer, and has been fast tracked by the FDA for expedited review in gastric cancer [40–42]. A Phase II trial of tesetaxel in advanced UC is planned.
Abraxane®
Albumin-bound paclitaxel (Abraxane®; Celgene Corporation, NJ, USA) has shown efficacy and superior tolerability in metastatic breast cancer. A Phase II two-stage trial of Abraxane in platinum-treated metastatic UC is ongoing [43]. Preliminary results in 14 patients, in which Abraxane was delivered at 260 mg/m2, revealed a clinical benefit rate of 71%, with ten patients experiencing either PR or stable disease [43]. The second stage of the trial is continuing with an accrual goal of 48 patients.
Eribulin
Eribulin is a synthetic analog of the black Pacific sea sponge macrolide halichondrin B [44]. The drug suppresses microtubule polymerization without affecting depolymerization and results in the formation of nonfunctional intracellular tubulin aggregates [44]. Preclinical studies established the antiproliferative effects of eribulin within multiple cancer cell lines, including prostate and breast, as well as in vivo efficacy against paclitaxel-resistant ovarian cell lines. Two Phase I trials defined the dose schedule and toxicity profile of eribulin in patients with advanced solid malignancies, and the most common adverse events included neutropenia and fatigue [45,46]. Neurotoxicity with eribulin was not a predominant toxicity in either trial. Preliminary results from a Phase II trial of eribulin in advanced UC were reported at the American Society of Clinical Oncology (ASCO) 2010 meeting (4–8 June 2010, IL, USA) [47]. In total, 40 patients were enrolled and received eribulin monotherapy at 1.4 mg/m2 on days 1 and 8 of a 3-week cycle. The ORR in 37 evaluable patients was 38% (95% CI: 23–54%), consisting of one CR and 14 PRs. In those patients who had received prior lines of chemotherapy, a comparable RR of 34% was observed. Median PFS and OS were 3.9 and 9.4 months, respectively, at a median follow-up of 19.8 months. A total of 20 patients experienced grade 3–4 neutropenia. Grade 1–2 sensory neuropathy was observed in 18 patients. In addition, because eribulin is minimally cleared through the kidney (<10%), a Phase I study examined the safety and efficacy of eribulin in patients with UC and depressed renal function [48]; 21 patients with moderate (≥40–59 ml/min) or severe (20–40 ml/min) renal dysfunction as defined by the Cockgroft–Gault formula were treated with escalating doses of eribulin with a maximum dose of 1.4 mg/m2. The maximum tolerated dose was defined as the highest dose achieved with no more than one dose-limiting toxicity. RR in 20 evaluable patients was 18% (95% CI: 5–41%) with three PRs. The median PFS and OS were 4.1 and 9.7 months, respectively, at a median follow-up of 11 months. Notably, patients with moderate renal dysfunction tolerated a dose of 1.4 mg/m2 with no dose-limiting toxicities observed. Among patients with severe renal dysfunction, one out of six patients receiving 1.4 mg/m2 experienced a dose-limiting toxicity of fatigue and weakness.
Novel targeted agents
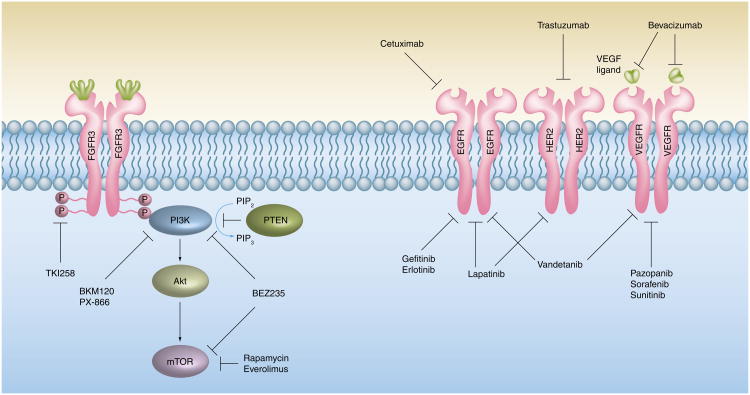
As research into the critical molecular pathways that drive bladder tumorigenesis progresses, new targets for selective inhibitor therapy will be identified. With the incorporation of targeted agents into the management of patients with multiple malignancies including NSCLC, breast, colon, glioblastoma and others, the utility of selective inhibitor therapy has been proven, both in combination with chemotherapy and as monotherapy [49–61]. These drugs target aberrantly activated pathways that are typically involved in regulating cellular proliferation, apoptosis and angiogenesis [51]. The major advantage of targeted therapy lies in its specificity and related favorable toxicity profile [50]. Table 2 summarizes trials involving select targeted therapies. Figure 1 depicts the major targets for commonly utilized and novel monoclonal antibodies and small molecule inhibitors.
Table 2.
Novel targeted agents in advanced urothelial Cancer.
| Author/study | Agent | Target | Regimen | Phase/n | RR (%) (95% CI) | Median OS (months) | Ref. |
|---|---|---|---|---|---|---|---|
| Philips et al. | Gefitinib | EGFR | With GC | II/54 | 42.6 (29.2–56.8) | 15.1 | [77] |
| MDACC (TX, USA) | Erlotinib | EGFR | Once daily | II/42 | Ongoing | Ongoing | |
| Dana–Farber Cancer Institute (MA, USA) | Vandetanib | EGFR, VEGFR-2, RET | Docetaxel ± vandetanib | II/140 | Ongoing | Ongoing | |
| University of Michigan (MI, USA) | Cetuximab | EGFR | GC ± cetuximab | II/81 | Ongoing | Ongoing | |
| Hussain et al. | Trastuzumab | HER2/neu | With G + carboplatin + paclitaxel | II/44 | 70 (55–83) | 14.1 | [68] |
| Hahn et al. | Bevacizumab | VEGF | With GC | II/45 | 67 (51–82) | NR | [88] |
| CALGB 90601 | With GC | III/500 | Ongoing | Ongoing | |||
| MSKCC (NY, USA) | With G + carboplatin | II/47 | Ongoing | Ongoing | |||
| MDACC | With dose-dense M-VAC | II/60 | Ongoing | Ongoing | |||
| Sridhar et al. | Sorafenib | VEGFR-2, -3, PDGFR-β, Raf | Twice daily | II/17 | None observed | 5.9 | [93] |
| Dreicer et al. | Twice daily | II/27 | None observed | 6.8 | [94] | ||
| Gallagher et al. | Sunitinib | VEGFR-2, PDGFR-β | Cohort A: daily for 4 out of 6 weeks Cohort B: daily | II/77 | 29 | A: 7.1 vs B: 6 (p = 0.4) | [96] |
| Mayo Clinic (MN, USA) | Pazopanib | VEGFR-1,-2,-3, PDGFR, c-kit | Once daily | II/32 | Ongoing | Ongoing | |
| Hoosier Oncology Group (IN, USA) | ASA404 | Endothelial cytoskeleton | With docetaxel | II/40 | Ongoing | Ongoing | |
| Cheung et al. | Vorinostat | HDAC | Twice daily | II/14 | None observed | 4.3 | [109] |
| Gomez-Abuin et al. | Bortezomib | 26S proteosome complex | Monotherapy | II/20 | None observed | 15 weeks | [113] |
| Rosenberg et al. | Monotherapy | II/25 | None observed | 5.7 | [114] |
CALGB: Cancer and Leukemia Group B; EGFR: EGF receptor; G: Gemcitabine; GC: Gemcitabine plus cisplatin; HDAC: Histone deacetylase enzyme; MDACC: MD Anderson Cancer Center; MSKCC: Memorial Sloan–Kettering Cancer Center; M-VAC: Methotrexate, vinblastine, doxorubicin and cisplatin; NR: Not reported; OS: Overall survival; PDGFR: PDGF receptor; RR: Response rate; VEGFR: VEGF receptor.
Figure 1. The PI3K–Akt pathway and select tyrosine kinase receptors with relevant pathway and receptor inhibitors.
EGFR: EGF receptor; FGFR: FGF receptor; P: Phosphate; PIP2: Phosphotidylinositol-4,5-bisphosphate; PIP3: Phosphotidylinositol-3,4,5-triphosphate; VEGFR: VEGF receptor.
EGF receptor inhibition
In UC, blockade of either the EGF receptor (EGFR) or its associated tyrosine kinase is under investigation. Overexpression of HER2, a member of the EGFR family, has been observed in 23–80% of bladder cancers, with increased levels of expression correlating with higher grade, increased metastatic potential and poor prognosis [62,63]. HER2 partners with EGFR and forms a heterodimer that binds EGF ligand with high affinity. Ligand binding stimulates the Ras–MAPK signal-transduction cascade, which in turn drives cell proliferation and migration [64]. Controversy exists as to the optimal method for detection of HER2 overexpression in UC, that is, immunohistochemical (IHC) analysis for protein overexpression, FISH to detect HER 2 gene amplification, or measurement of shed HER2 extracellular domain protein through an ELISA. Furthermore, while HER2 overexpression in breast cancer is characterized by concomitant genetic amplification, HER2-positive bladder tumors typically manifest protein overexpression alone with a much lower incidence of HER2 genetic amplification. A large retrospective study by Lae et al. attempted to define the incidence of both HER2 gene and protein overexpression by applying the same diagnostic criteria currently utilized in breast cancer to 1005 invasive paraffin-embedded UC samples [65]. In total, 93 tumors (9.2%) revealed either 2+ or 3+ HER2 protein expression by IHC; all samples with 3+ protein overexpression contained gene amplification by FISH compared with none of the samples with 2+ overexpression. By contrast, there is a strong correlation between HER2 protein overexpression by IHC and underlying gene amplification by FISH in breast cancer, suggesting that the majority of HER 2 gene amplifications in breast cancer result in HER2 protein overexpression. Interestingly, ten out of ten lymph node metastases and matched primary tumors had 3+ HER2 protein overexpression (FISH testing was not performed in these samples), consistent with a previously described association between HER2 overexpression and metastatic potential [66].
Monoclonal antibodies
Trastuzumab
The humanized monoclonal anti-HER2 antibody trastuzumab has demonstrated significant activity in HER2-positive breast cancer, both in the metastatic and adjuvant settings [67]. In a multicenter Phase II NCI-198 trial, 44 patients with advanced UC and overexpression of HER2 by either IHC, FISH or serum HER2 extracellular domain (ECD) testing received chemotherapy consisting of carboplatin, paclitaxel and gemcitabine in combination with trastuzumab [68]. Metastatic disease was present in all treated patients and 32.6% of patients had received prior chemotherapy. The primary end point of this study was cardiac toxicity rate while secondary end points included RR, time to disease progression and survival. Ten patients (22.7%) experienced grades 1–3 cardiac toxicities. The RR was 70% (31 patients), with five CRs and 26 PRs. The median time to progression was 9.3 months while median survival was 14.1 months (95% CI: 11.5–17.1 months); median survival was 15.8 months (95% CI: 11.4–24.3 months) for patients with HER2 overexpression. Interestingly, 75% of patients with 3+ HER2 over expression by IHC exhibited a response, compared with 67% in those with 2+ IHC overexpression; furthermore, 82% of patients with FISH-positive overexpression experienced a response versus 67% of FISH-negative patients. These results suggest that RRs may be highest in those patients with the greatest degree of HER2 overexpression, similar to findings in HER2-positive breast cancers [69]. Of note, patients with HER2 overexpression also had a higher median number of metastatic sites (two sites vs one site; p = 0.014), a trend toward increased visceral and bony metastases, and the presence of two or more metastatic sites was more common (51 vs 31%; p = 0.051), thus comprising a high-risk baseline population. Prospective measurement of HER2 expression using IHC, FISH or detection of serum HER2 ECD levels revealed that 52% of patients displayed HER2 overexpression. In total, 93% of patients with HER2 overexpression were detected by IHC, while only a subset of these patients were positive by FISH or serum testing (26 and 23%, respectively). Again, in contrast to the concordant HER2 gene amplification found in breast cancer, these results concur with the finding that the majority of HER2 overexpression in UC occurs without underlying genetic amplification [70].
Cetuximab
Cetuximab is a recombinant humanized murine monoclonal antibody that targets the extracellular domain of the EGFR (HER1), leading to inhibition of downstream mitogenic signal transduction pathways typically stimulated by ligand binding. Its efficacy in metastatic colorectal cancer, as well as squamous cell carcinoma of the head and neck, has led to its FDA approval for these diseases [71,72]. Cetuximab possesses antiproliferative activity at least partly through an antiangiogenic mechanism in mouse models of UC, and this effect is synergistic with paclitaxel [73,74]. A randomized Phase II trial coordinated by the University of Michigan (MI, USA) is currently accruing in which patients with metastatic, locally recurrent or unresectable UC are administered GC either alone or in combination with cetuximab.
Tyrosine kinase inhibitors
Gefitinib
Gefitinib, an oral inhibitor of the tyrosine kinase activity of EGFR, has shown significant clinical activity in EGFR mutant NSCLC [75]. Gefitinib is also effective at inhibiting cell proliferation in EGFR-expressing human bladder cancer cell lines with addition of drug leading to suppression of both Akt and ERK phosphorylation [76]. A Phase II Cancer and Leukemia Group B (CALGB) trial investigated the efficacy of gefitinib in combination with gemcitabine and cisplatin in patients with advanced UC [77]. This combination of agents had been previously shown to have synergistic effects compared with chemotherapy alone with GC in mouse xenograft models. Such synergy was thought to be related to blockade of EGFR signal transduction with the concomitant antiproliferative effects of chemotherapeutic agents. The initial cohort of 25 patients were administered a fixed dose rate infusion of gemcitabine 10 mg/m2/min with cisplatin plus gefitinib. Significant grade 4 nonhematologic toxicities, including hyponatremia, hyperuricemia, dyspnea, and venous thromboembolism, as well as death from internal carotid artery thrombosis and urosepsis occurred, leading to accrual discontinuation and subsequent modification of the gemcitabine administration to a 30-min infusion. A total of 54 patients were evaluated with an ORR of 42.6% (95% CI: 29.2–56.8%) consisting of seven CRs and 16 PRs. Median OS was 15.1 months (95% CI: 11.1–21.7 months) and median time to progression was 7.4 months (95% CI: 5.6–9.2 months). A follow-up analysis at 39.5 months revealed that 51 patients had died. The OS and time to progression with the addition of geftinib was not significantly different compared with historical gemcitabine and cisplatin alone [7]. There are no ongoing clinical trials evaluating geftinib in the metastatic setting.
Erlotinib & lapatinib
The oral EGFR tyrosine kinase inhibitor (TKI) erlotinib has been shown to inhibit the proliferation of UC cells in vitro[78]. This drug is currently being investigated in the neoadjuvant setting in patients with locally advanced UC. Lapatinib, a dual EGFR and HER2 TKI, has shown preclinical anti-tumor activity in multiple bladder cancer cell lines [79]. A single-arm Phase II trial investigated the efficacy of lapatinib in patients with platinum-refractory UC. Out of 59 enrolled patients, 34 were evaluable for response. An ORR of greater than 10% was seen in 1.7% of patients (95% CI: 0–9.1%). Stable disease was observed in 18 patients (31%; 95% CI: 19–44%). OS was 17.9 weeks and time to progression 8.6 weeks. Of note, OS was significantly increased with EGFR or HER2 overexpression (p = 0.0001) [80]. A Phase II/III trial is currently examining the role of maintenance lapatinib in patients with HER1 and/or HER2-positive advanced UC following chemotherapy. Eligibility criteria include an objective response or stable disease by Response Evaluation Criteria in Solid Tumors (RECIST) after administration of first-line chemotherapy, as well as evidence of HER1 and/or HER2 overexpression by FISH or IHC. Patients are randomized to receive lapatinib 1500 mg daily or placebo until disease progression.
Antiangiogenesis agents
Angiogenesis plays a crucial role in tumor development and maintenance, as well as metastasis. In metastatic bladder cancer, the presence of high serum VEGF levels corresponds to decreased disease-free survival [81]. Microvessel density, a surrogate marker of angiogenesis in UC, is also associated with a poor prognosis, and both microvessel density and VEGF mRNA levels were defined as independent predictors of metastasis and disease recurrence in 51 patients who were treated with neoadjuvant M-VAC chemotherapy and cystectomy [82,83]. Targeting tumor dependence on angiogenesis with antiangiogenic therapies in the form of monoclonal antibodies and small-molecule inhibitors has significantly expanded treatment options for patients with advanced solid tumor malignancies.
Monoclonal antibodies
Bevacizumab
Bevacizumab is a recombinant humanized monoclonal antibody targeting all isoforms of circulating VEGF. It has led to an improvement in outcome in advanced colorectal cancer, glioblastoma, NSCLC, breast cancer and renal cell cancer [84–87]. In a Phase II trial led by the Hoosier Oncology Group, bevacizumab was administered concomitantly with GC as first-line therapy in 43 patients with metastatic UC [88]. Patients initially received gemcitabine at a dose of 1250 mg/m2. However, after seven thromboembolic events occurred in the first 17 accrued patients, the gemcitabine dose was reduced to 1000 mg/m2. Preliminary results presented at the ASCO Annual Meeting 2009 revealed an ORR of 67% (95% CI: 51–82%), with six CRs and 18 PRs. In total, 47% of patients experienced grade 3–4 hematologic toxicity (thrombocytopenia and neutropenic fever) while grade 3–4 nonhematologic toxicity was present in 77% of patients (including deep vein thrombosis/pulmonary embolism, CNS hemorrhage, proteinuria and hypertension). These results suggest the potential for an improvement in response with the addition of bevacizumab complicated by significant treatment-related toxicities. A CALGB Phase III randomized trial of GC with or without bevacizumab in the first-line treatment of metastatic disease is ongoing. Additional trials currently accruing include a Phase II study of first-line gemcitabine, carboplatin and bevacizumab in patients with advanced UC who are not candidates for cisplatin (MSKCC), as well as a Phase II trial of dose-dense M-VAC combined with bevacizumab in the neoadjuvant setting (MD Anderson Cancer Center, TX, USA) [89].
Tyrosine kinase inhibitors
The multikinase inhibitors sorafenib, sunitinib and, more recently, pazopanib have revolutionized the treatment of meta-static renal cell carcinoma (RCC) and are also under investigation in advanced UC [90–92].
Sorafenib
Sorafenib inhibits Raf kinase, PDGF receptor (PDGFR)-β, VEGF receptor (VEGFR)-2, and VEGFR-3, while sunitinib blocks the activity of VEGFR-2 and PDGFR-β. The efficacy of first-line sorafenib in advanced bladder cancer was tested in a Phase II multicenter study through the Princess Margaret Hospital (ON, Canada) and the National Cancer Institute of Canada for which preliminary results suggested no significant response to therapy in 14 evaluable patients [93]. A similar Phase II ECOG trial examined sorafenib monotherapy in patients with advanced UC who had progressed on platinum-based chemotherapy and also revealed minimal activity [94].
Sunitinib
Sunitinib possesses antiproliferative activity against human bladder cancer cell lines and this activity is synergistic with the addition of cisplatin in murine xenografts [95]. Based upon these pre-clinical data, a Phase II study was performed at MSKCC in which sunitinib was administered to 77 patients with advanced UC in the second-line setting. Two drug-administration schedules were tested in separate cohorts: the initial cohort A received 50 mg once daily for 4 consecutive weeks out of a 6-week cycle, while cohort B received 37.5 mg once daily continuously. The primary end points were RR and safety while secondary end points included time to progression and OS. Three patients (7%) displayed a PR and 11 had stable disease in cohort A by RECIST criteria; in cohort B, one PR (3%) was observed and 15 patients had stable disease. Overall, a reduction in measurable disease was observed in 29 patients, with six patients experiencing greater than 20% reduction [96]. Clinical benefit from sunitinib (defined as PR or standard deviation lasting longer than 3 months) was observed in 22 patients (29%). Although response was modest, the significant number of patients with stable disease underscores the potential for targeted inhibition of the VEGF pathway in prior platinum-treated UC. A randomized double-blind, placebo-controlled Phase II trial is investigating the efficacy of maintenance sunitinib following 4–6 cycles of standard chemotherapy to determine if sunitinib decreases progression rates in patients with advanced UC who have obtained stable disease or better after standard chemotherapy [97].
Pazopanib
Pazopanib inhibits VEGFR-1, -2, -3, c-kit and PDGFR [98]. In a Phase III randomized controlled study of pazopanib versus placebo in metastatic RCC, a significant prolongation of PFS and RR was observed in patients receiving pazopanib [92]. As such, its antiangiogenic effects in metastatic UC are currently being evaluated in an ongoing Phase II trial. The primary end point is ORR while secondary end points include time to progression, OS, and frequency and type of adverse events.
Vandetanib
Vandetanib is an oral TKI that blocks the activity of VEGFR-2 and EGFR, as well as RET [99,100]. This agent has been shown to inhibit the growth of preclinical models of NSCLC [101]. Furthermore, a large Phase III trial in locally advanced or meta-static NSCLC demonstrated the superiority of vandetanib plus docetaxel in extending PFS compared with docetaxel alone [102]. A Phase II trial is currently accruing patients with advanced UC in which individuals are randomized to receive docetaxel alone or docetaxel plus vandetanib. The primary outcome of the study is PFS and secondary outcomes include OS, ORR, and incidence of adverse events and toxicities grade 3 or higher.
Vascular-disrupting agents
Representing a distinct class of anti-tumor drugs, the vascular-disrupting agents (VDAs) destroy existing tumor-associated vasculature, leading to significant tumor necrosis and cell death [103,104]. Preclinical findings suggest that exposure to VDAs leads to selective endothelial cell apoptosis through intracellular cytoskeletal disruption [103,105]. These agents differ from anti angiogenic agents, which target new blood vessel growth, thereby inhibiting further tumor growth and metastasis.
ASA404
ASA404, a novel VDA, demonstrated promising preclinical activity against multiple tumor cell lines as well as synergy when administered with taxanes [106,107]. Unfortunately, a randomized Phase III trial investigating this compound in the first-line setting for advanced NSCLC (ATTRACT-1) was recently discontinued following a planned interim analysis that showed no benefit with the addition of the drug to standard platinum-based therapy [201]. However, a Phase II trial of ASA404 in combination with docetaxel in patients with metastatic UC in the second-line setting is currently accruing.
Epigenetic-modulating agents
Histone deacetylase inhibitors
Tumor suppressors such as p53 and Rb serve as checkpoints to neoplastic transformation by halting cell proliferation or inducing apoptosis in the face of irreparable genetic damage. Inactivation of tumor suppressors with subsequent tumor development can occur through multiple mechanisms, including gene deletion, loss of heterozygosity, or epigenetic transcriptional silencing via aberrant promoter methylation or histone deacetylation. This last process is mediated by histone deacetylase enzymes (HDACs) and leads to a tighter association between packaging histones and DNA, preventing access to transcription-promoting sequences. The HDAC inhibitors vorinostat and romidepsin have been approved for the treatment of cutaneous T-cell lymphoma.
Vorinostat
Preclinical data with suberoylanilide hydroxamic acid (SAHA), another HDAC inhibitor, has demonstrated inhibition of proliferation of the T24 human bladder cancer cell line [108]. A Phase II trial of second-line SAHA in 14 patients with locally recurrent or metastatic UC was performed through the California Cancer Consortium [109]. SAHA was administered orally at a dose of 200 mg twice daily until disease progression or toxicity. Three patients achieved stable disease while eight experienced progression. The median disease-free survival was 1.1 months and OS was 4.3 months. Two on-study deaths occurred, five patients experienced either grade 4 or 5 toxicity, and four patients experienced grade 3 toxicity. The study was closed to further accrual, with the conclusion that SAHA did not display significant efficacy in the second-line setting and was associated with toxicity.
Demethylating agents
Decitabine
Demethylating agents are also being studied in the treatment of UC. 5-aza-2′-deoxycytidine, or decitabine, is a DNA methyltransferase inhibitor that has shown preclinical synergistic activity in combination with cisplatin against five human bladder cancer cell lines [110]. Early-phase clinical trials will measure the efficacy of decitabine in patients with UC.
Proteasome inhibitors
Bortezomib
Bortezomib is an inhibitor of the 26S proteasome complex that degrades ubiquitinated proteins. Proteasome blockade sub sequently leads to cell cycle arrest and apoptosis. Exposure of UC cell lines to combination bortezomib and gemcitabine resulted in inhibition of proliferation as well as stimulation of apoptotic pathways [111,112]. Bortezomib has been tested in two Phase II trials in patients with advanced UC. A total of 20 eligible patients were enrolled through the Princess Margaret Hospital and the University of Chicago (IL, USA) Phase II consortia and were treated twice weekly for 2 out of 3 weeks with infusional bortezomib [113]. No objective responses were detected, the median time to progression was 8.1 weeks (95% CI: 6.4–9), and median OS was approximately 15 weeks (95% CI: 3.6–NA). A total of 15 patients experienced either grade 3 or 4 toxicities. In a sub sequent trial conducted by the CALGB, 25 patients with advanced UC refractory to prior chemotherapy were administered bortezomib [114]. No objective responses were noted, the median time to progression was 1.4 months (95% CI: 1.1–2 months) and median survival time was 5.7 months (95% CI: 3.6–8.4 months). Based on these studies, bortezomib does not appear to possess significant activity against UC in the second-line setting.
Other TKIs
Fibroblast growth factor receptor-3 (FGFR3) is a receptor tyrosine kinase that is mutated in up to 75% of low-grade, non- muscle-invasive bladder tumors and approximately 20% of muscle-invasive lesions [115,116]. Hotspot mutations within exons 7, 10 and 15 account for the majority of alterations and characteristically lead to constitutive dimerization and subsequent activation of the kinase [117]. FGFR3 is thought to transduce extracellular mitogenic signals using both the MAPK and PI3K–Akt pathways, resulting in increased cell growth, proliferation and survival [118]. Interestingly, mutations within both FGFR3 and TP53 rarely coexist, suggesting that urothelial tumors possessing FGFR3 mutations may be uniquely dependent on the activated FGFR3 for maintenance of a neoplastic phenotype [117]. The relatively high frequency of FGFR3 mutations in UC has generated substantial interest in the development of targeted inhibitors against the activated enzyme.
Tyrosine kinase inhibitor 258
Tyrosine kinase inhibitor 258 is a multi-targeted TKI that blocks the tyrosine kinase activity of FGFR, VEGFR, PDGFR-β and Flt-3, and has been shown in preclinical studies to have both direct cytotoxic and antiangiogenic activity [119]. Phase I studies have proven the safety and defined the maximum tolerated dose of this drug [120]. It will be investigated in an upcoming Phase II two-stage clinical trial in patients with advanced platinum-refractory UC who are stratified based upon the FGFR3 mutation status of their tumors. A total of 20 patients in each arm will receive 500 mg once daily of drug on a 5 days on and 2 days off schedule. The primary end point of the study is ORR with secondary end points including PFS and safety.
PI3K–Akt pathway inhibitors
A wide spectrum of PI3K–Akt pathway mutations has been reported in UC. PI3K is a heterodimeric lipid kinase that, upon activation by receptor tyrosine kinases, catalyzes the conversion of phosphotidylinositol-4,5-bisphosphate (PIP2) to phosphotidylino-sitol-3,4,5-triphosphate (PIP3) [121]. PIP recruits multiple proteins to the plasma membrane including PDK1 and Akt. Akt is activated by phosphorylation at threonine 308 and stimulates numerous downstream pathways that upregulate cell growth and proliferation and suppress apoptosis [122]. For example, activated Akt phosphorylates and inhibits the GTPase-activating protein tuberous sclerosis complex-2 (TSC2), a molecule that constitutively represses the Ras homolog enriched in brain (Rheb) protein. Subsequent activation of Rheb results in stimulation of mTOR, a key promoter of translation in response to nutrient levels [122,123]. mTOR exerts its effects through phosphorylation and activation of S6 kinase and the eukaryotic initiation factor 4E-binding protein (4EBP1) [123].
A number of alterations of this pathway can result in unchecked cell growth and proliferation in UC. These include mutations within the PIK3CA gene that encodes for the p110α catalytic subunit of PI3K (seen in up to 27% of some low-grade tumor series) as well as loss of function mutations of PTEN, the lipid phosphatase that negatively regulates PI3K-mediated conversion of PIP2 to PIP3[124]. Homozygous deletions as well as downregulation of PTEN expression through copy number loss are frequently found in invasive UC concomitant with TP53 mutations [124]. Mutations of the PIK3CA and AKT genes can lead to increased activation of the pathway [118]. Inactivation of TSC1 leads to constitutive activation of mTOR, resulting in uncontrolled cell growth and proliferation, and up to 14.5% of UC tumors contain alterations within TSC1 [124]. Ras mutations can also lead to in appropriate PI3K–Akt pathway stimulation since Ras is known to initiate PI3K-mediated signaling [125].
While a plethora of possible aberrations exist that can lead to inappropriate activation of the PI3K–Akt pathway, these aberrations may also serve as targets for inhibition. A number of PI3K pathway inhibitors are currently in development and include PX-866 and BKM120, which block the activity of PI3K alone, as well as BEZ235, a dual PI3K/mTOR inhibitor currently being tested in a Phase I/II trial [126]. Two major classes of PI3K inhibitor exist, namely the non-isoform-specific and the isoform-specific drugs. Whether pan-PI3K inhibition leads to an increased incidence of toxicities is unknown at this time but this issue will need be evaluated in the ongoing Phase 1 trials of these agents. Preliminary data suggest that pathway inhibition, regardless of the class of drug utilized, results mainly in cytostasis as opposed to cytotoxicity [126–128]. Figure 1 depicts inhibitors of the PI3K–Akt pathway that are currently being investigated.
mTOR inhibitors
Mammalian target of rapamycin inhibition has been shown to be highly effective in RCC, and the rapalog temsirolimus is now FDA approved in the management of this disease [129]. More recently, everolimus gained FDA approval for use in the second-line setting following failure of sorafenib or sunitinib in RC [130]. The efficacy of everolimus in advanced UC is currently being investigated through a Phase II trial in which patients with progressive, metastatic disease receive 10 mg of drug orally daily until progression. The primary end point of this study is 2-month PFS rate as well as characterization of toxicities associated with everolimus. Secondary outcome measures include RR and assessment of mTOR activation through IHC detection of markers of mTOR activation, namely phosphorylated S6 and 4EBP1, in pretreatment tissue. Prior data in the form of a tissue microarray comprising 92 muscle-invasive UC specimens revealed overexpression of both phospho-S6 and 4EBP1 with significant correlation of expression within the mTOR pathway, suggesting that this pathway is active in subsets of UC patients and may be an effective target for small-molecule inhibition [131].
Expert commentary
Systemic chemotherapy consisting of a platinum-based regimen remains the standard of care for patients with advanced UC. However, with a median survival in the metastatic setting of approximately 12–15 months and RRs for single-agent therapy in the second-line setting ranging from 10 to 20%, novel therapies are desperately needed in this disease.
Five-year view
While significant discoveries have been made regarding the molecular pathogenesis of UC, no biologic agents have yet been approved either as monotherapy or in combination with traditional chemotherapy in the treatment of metastatic or recurrent UC, although numerous clinical trials evaluating these agents, as well as new cytotoxic therapies with improved tolerance, are underway. A more complete understanding of the complex biology underlying urothelial carcinogenesis will most certainly lead to the development of more effective rational therapies in this disease.
Key issues.
Systemic chemotherapy with a platinum-based regimen is the current standard of care for advanced urothelial cancer (UC); however, approximately half of patients with advanced disease are not candidates for cisplatin-based treatment regimens and represent a true unmet medical need.
While both the methotrexate, vinblastine, doxorubicin and cisplatin, and gemcitabine plus cisplatin regimens possess similar efficacy in patients with advanced UC, gemcitabine plus cisplatin is better tolerated with fewer side effects and has become a standard of care for the first-line management of patients with metastatic UC who are candidates for cisplatin-based chemotherapy.
Second-line chemotherapy agents and regimens have shown only modest activity in advanced UC and there is no available US FDA-approved agent.
A two-variable prognostic model that correlates with shortened median survival in the metastatic setting includes Karnofsky performance status of 80% or less and the presence of visceral metastases. The proportion of patients in the associated risk categories may have a significant impact on the results of clinical trials.
Biomarkers that predict response to both standard cytotoxic agents and novel targeted therapeutics are desperately needed.
Identification of the key signaling pathways driving bladder tumorigenesis will open new avenues for the use of targeted therapies with the potential for improved toxicity profiles compared with standard chemotherapy.
Ongoing clinical trials evaluating the efficacy of selective kinase inhibitors and other targeted agents in UC patients whose tumors possess the genetic profile predicted to be most likely to respond will ultimately lead to the development of rational combination strategies for patients in whom combinations of mutations drive malignant transformation.
Numerous clinical trials are underway to investigate the efficacy of novel cytotoxic agents and targeted therapies in advanced UC, and clinicians must enroll patients in these trials whenever possible.
Footnotes
Financial & competing interests disclosure: The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Cookson MS, Herr HW, Zhang ZF, et al. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol. 1997;158(1):62–67. doi: 10.1097/00005392-199707000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Grossman HB, O'Donnell MA, Cookson MS, Greenberg RE, Keane TE. Bacillus Calmette–Guérin failures and beyond: contemporary management of non-muscle-invasive bladder cancer. Rev Urol. 2008;10(4):281–289. [PMC free article] [PubMed] [Google Scholar]
- 4••.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666–675. doi: 10.1200/JCO.2001.19.3.666. Large, retrospective experience supporting the aggressive surgical management of bladder cancer and demonstrating the influence of pathologic stage and lymph node involvement on outcome in patients with localized disease. [DOI] [PubMed] [Google Scholar]
- 5.Milowsky MI, Stadler WM, Bajorin DF. Integration of neoadjuvant and adjuvant chemotherapy and cystectomy in the treatment of muscle-invasive bladder cancer. BJU Int. 2008;102(9 Pt B):1339–1344. doi: 10.1111/j.1464-410X.2008.07980.x. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg CN, Yagoda A, Scher HI, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol. 1985;133(3):403–407. doi: 10.1016/s0022-5347(17)48996-8. [DOI] [PubMed] [Google Scholar]
- 7••.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, Phase III study. J Clin Oncol. 2000;18(17):3068–3077. doi: 10.1200/JCO.2000.18.17.3068. The Phase III trial that demonstrated similar outcomes but reduced toxicity with the GC regimen as compared to the M-VAC regimen in the first-line setting in patients with advanced UC and thereby establishing GC as an alternative with less toxicity to the M-VAC regimen. [DOI] [PubMed] [Google Scholar]
- 8.Hillcoat BL, Raghavan D, Matthews J, et al. A randomized trial of cisplatin versus cisplatin plus methotrexate in advanced cancer of the urothelial tract. J Clin Oncol. 1989;7(6):706–709. doi: 10.1200/JCO.1989.7.6.706. [DOI] [PubMed] [Google Scholar]
- 9.Harker WG, Meyers FJ, Freiha FS, et al. Cisplatin, methotrexate, and vinblastine (CMV): an effective chemotherapy regimen for metastatic transitional cell carcinoma of the urinary tract. A Northern California Oncology Group study. J Clin Oncol. 1985;3(11):1463–1470. doi: 10.1200/JCO.1985.3.11.1463. [DOI] [PubMed] [Google Scholar]
- 10.Loehrer PJ, Sr, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1992;10(7):1066–1073. doi: 10.1200/JCO.1992.10.7.1066. [DOI] [PubMed] [Google Scholar]
- 11.Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a Phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1997;15(7):2564–2569. doi: 10.1200/JCO.1997.15.7.2564. [DOI] [PubMed] [Google Scholar]
- 12••.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17(10):3173–3181. doi: 10.1200/JCO.1999.17.10.3173. Retrospective analysis that defined the prognostic risk factors predicting survival in patients with metastatic UC receiving first-line chemotherapy and which can be used to stratify patients in clinical trials. [DOI] [PubMed] [Google Scholar]
- 13.Roth BJ, Dreicer R, Einhorn LH, et al. Significant activity of paclitaxel in advanced transitional-cell carcinoma of the urothelium: a Phase II trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 1994;12(11):2264–2270. doi: 10.1200/JCO.1994.12.11.2264. [DOI] [PubMed] [Google Scholar]
- 14.McCaffrey JA, Hilton S, Mazumdar M, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol. 1997;15(5):1853–1857. doi: 10.1200/JCO.1997.15.5.1853. [DOI] [PubMed] [Google Scholar]
- 15.Stadler WM, Kuzel T, Roth B, Raghavan D, Dorr FA. Phase II study of single-agent gemcitabine in previously untreated patients with metastatic urothelial cancer. J Clin Oncol. 1997;15(11):3394–3398. doi: 10.1200/JCO.1997.15.11.3394. [DOI] [PubMed] [Google Scholar]
- 16.Bamias A, Aravantinos G, Deliveliotis C, et al. Docetaxel and cisplatin with granulocyte colony-stimulating factor (G-CSF) versus MVAC with G-CSF in advanced urothelial carcinoma: a multicenter, randomized, Phase III study from the Hellenic Cooperative Oncology Group. J Clin Oncol. 2004;22(2):220–228. doi: 10.1200/JCO.2004.02.152. [DOI] [PubMed] [Google Scholar]
- 17.Bajorin DF, McCaffrey JA, Hilton S, et al. Treatment of patients with transitional-cell carcinoma of the urothelial tract with ifosfamide, paclitaxel, and cisplatin: a Phase II trial. J Clin Oncol. 1998;16(8):2722–2727. doi: 10.1200/JCO.1998.16.8.2722. [DOI] [PubMed] [Google Scholar]
- 18.Bellmunt J, Guillem V, Paz-Ares L, et al. Phase 1– study of paclitaxel, cisplatin, and gemcitabine in advanced transitional-cell carcinoma of the urothelium Spanish Oncology Genitourinary Group. J Clin Oncol. 2000;18(18):3247–3255. doi: 10.1200/JCO.2000.18.18.3247. [DOI] [PubMed] [Google Scholar]
- 19.Bellmunt J, Albanell J, Gallego OS, et al. Carboplatin, methotrexate, and vinblastine in patients with bladder cancer whowere ineligible for cisplatin-based chemotherapy. Cancer. 1992;70(7):1974–1979. doi: 10.1002/1097-0142(19921001)70:7<1974::aid-cncr2820700727>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Boccardo F, Pace M, Guarneri D, et al. Carboplatin, methotrexate, and vinblastine in the treatment of patients with advanced urothelial cancer. A Phase II trial. Cancer. 1994;73(7):1932–1936. doi: 10.1002/1097-0142(19940401)73:7<1932::aid-cncr2820730726>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 21.Bajorin DF, McCaffrey JA, Dodd PM, et al. Ifosfamide, paclitaxel, and cisplatin for patients with advanced transitional cell carcinoma of the urothelial tract: final report of a Phase II trial evaluating two dosing schedules. Cancer. 2000;88(7):1671–1678. [PubMed] [Google Scholar]
- 22.Hussain M, Vaishampayan U, Du W, Redman B, Smith DC. Combination paclitaxel, carboplatin, and gemcitabine is an active treatment for advanced urothelial Cancer. J Clin Oncol. 2001;19(9):2527–2533. doi: 10.1200/JCO.2001.19.9.2527. [DOI] [PubMed] [Google Scholar]
- 23.Pectasides D, Glotsos J, Bountouroglou N, et al. Weekly chemotherapy with docetaxel, gemcitabine and cisplatin in advanced transitional cell urothelial Cancer: a Phase II trial. Ann Oncol. 2002;13(2):243–250. doi: 10.1093/annonc/mdf017. [DOI] [PubMed] [Google Scholar]
- 24••.Bellmunt J, von der Maase H, Mead GM, et al. Randomized Phase III study comparing paclitaxel/cisplatin/gemcitabine (PCG) and gemcitabine/cisplatin (GC) in patients with locally advanced (LA) or metastatic (M) urothelial Cancer without prior systemic therapy; EORTC30987/Intergroup Study. Proc Am Soc Clin Oncol. 2007;25:18S. (Abstract LBA5030). Randomized clinical trial in patients with advanced UC comparing triplet chemotherapy with PCG compared with doublet chemotherapy with GC demonstrating no significant survival benefit with the triplet regimen. [Google Scholar]
- 25.Gallagher DJ, Milowsky MI, Bajorin DF. Advanced bladder Cancer: status of first-line chemotherapy and the search for active agents in the second-line setting. Cancer. 2008;113(6):1284–1293. doi: 10.1002/cncr.23692. [DOI] [PubMed] [Google Scholar]
- 26••.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28(11):1850–1855. doi: 10.1200/JCO.2009.25.4599. Identification and validation of prognostic risk factors, which predict for overall survival in platinum-refractory patients. [DOI] [PubMed] [Google Scholar]
- 27.Bellmunt J, Paz-Ares L, Cuello M, et al. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder Cancer patients receiving cisplatin-based chemotherapy. Ann Oncol. 2007;18(3):522–528. doi: 10.1093/annonc/mdl435. [DOI] [PubMed] [Google Scholar]
- 28••.Smith SC, Baras AS, Lee JK, Theodorescu D. The COXEN principle: translating signatures of in vitro chemosensitivity into tools for clinical outcome prediction and drug discovery in Cancer. Cancer Res. 2010;70(5):1753–1758. doi: 10.1158/0008-5472.CAN-09-3562. Description of the gene-based COXEN model as a novel platform for predicting chemosensitivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodin S, Kane MP, Rubin EH. Epothilones: mechanism of action and biologic activity. J Clin Oncol. 2004;22(10):2015–2025. doi: 10.1200/JCO.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Kowalski RJ, Giannakakou P, Hamel E. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol®) J Biol Chem. 1997;272(4):2534–2541. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 31.Dreicer R, Li S, Manola J, et al. Phase 2 trial of epothilone B analog BMS-247550 (ixabepilone) in advanced carcinoma of the urothelium (E3800): a trial of the Eastern Cooperative Oncology Group. Cancer. 2007;110(4):759–763. doi: 10.1002/cncr.22839. [DOI] [PubMed] [Google Scholar]
- 32.Paz-Ares L, Tabernero J, Moyano A. A Phase II study of the multi-targeted antifolate, MTA (LY231514), in patients with advanced transitional cell carcinoma (TCC) of the bladder. Proc Am Soc Clin Oncol. 1998 Abstract 1307. [Google Scholar]
- 33.Sweeney CJ, Roth BJ, Kabbinavar FF, et al. Phase II study of pemetrexed for second-line treatment of transitional cell Cancer of the urothelium. J Clin Oncol. 2006;24(21):3451–3457. doi: 10.1200/JCO.2005.03.6699. [DOI] [PubMed] [Google Scholar]
- 34.Galsky MD, Mironov S, Iasonos A, et al. Phase II trial of pemetrexed as second-line therapy in patients with metastatic urothelial carcinoma. Invest New Drugs. 2007;25(3):265–270. doi: 10.1007/s10637-006-9020-9. [DOI] [PubMed] [Google Scholar]
- 35.Bennouna J, Delord JP, Campone M, Nguyen L. Vinflunine: a new microtubule inhibitor agent. Clin Cancer Res. 2008;14(6):1625–1632. doi: 10.1158/1078-0432.CCR-07-2219. [DOI] [PubMed] [Google Scholar]
- 36.Culine S, Theodore C, De Santis M, et al. A Phase II study of Vinflunine in bladder Cancer patients progressing after first-line platinum-containing regimen. Br J Cancer. 2006;94(10):1395–1401. doi: 10.1038/sj.bjc.6603118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaughn DJ, Srinivas S, Stadler WM, et al. Vinflunine in platinum-pretreated patients with locally advanced or metastatic urothelial carcinoma: results of a large Phase 2 study. Cancer. 2009;115(18):4110–4117. doi: 10.1002/cncr.24460. [DOI] [PubMed] [Google Scholar]
- 38.Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of Vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitionalcell carcinoma of the urothelial tract. J Clin Oncol. 2009;27(27):4454–4461. doi: 10.1200/JCO.2008.20.5534. [DOI] [PubMed] [Google Scholar]
- 39.Metzger-Filho O, Moulin C, de Azambuja E, Ahmad A. Larotaxel: broadening the road with new taxanes. Expert Opin Invest Drugs. 2009;18(8):1183–1189. doi: 10.1517/13543780903119167. [DOI] [PubMed] [Google Scholar]
- 40.Moore MR, Jones C, Harker G. Phase II trial of DJ-927, an oral tubulin depolymerization inhibitor, in the treatment of metastatic colorectal Cancer. Proc Am Soc Clin Oncol. 2006;24:168s. Abstract 3591. [Google Scholar]
- 41.Szczesna A, Milanowski E, Juhász E. A Phase II study of DJ-927 administered orally once every three weeks as a second line therapy to subjects with locally advanced or metastatic nonsmall cell lung Cancer after failure of platinum-based non-taxane regimen. Proc Am Soc Clin Oncol. 2006;24:667s. Abstract 17006. [Google Scholar]
- 42.Evans T, Dobrila R, Berardi R. A Phase II study of DJ-927 as second-line therapy in patients (pts) with advanced gastric Cancer (GC) who have failed a 5-FU non taxane based regimen. Proc Am Soc Clin Oncol. 2006;24:18s. Abstract 4081. [Google Scholar]
- 43.Sridhar SS, Canil CM, Eisen A, et al. A Phase II study of single agent abraxane as second-line therapy in patients with advanced urothelial carcinoma. J Clin Oncol. 2009;27(Suppl) Abstract e16058. [Google Scholar]
- 44.Jimeno A. Eribulin: rediscovering tubulin as an antiCancer target. Clin Cancer Res. 2009;15(12):3903–3905. doi: 10.1158/1078-0432.CCR-09-1023. [DOI] [PubMed] [Google Scholar]
- 45.Goel S, Mita AC, Mita M, et al. A Phase I study of eribulin mesylate (E7389), a mechanistically novel inhibitor of microtubule dynamics, in patients with advanced solid malignancies. Clin Cancer Res. 2009;15(12):4207–4212. doi: 10.1158/1078-0432.CCR-08-2429. [DOI] [PubMed] [Google Scholar]
- 46.Tan AR, Rubin EH, Walton DC, et al. Phase I study of eribulin mesylate administered once every 21 days in patients with advanced solid tumors. Clin Cancer Res. 2009;15(12):4213–4219. doi: 10.1158/1078-0432.CCR-09-0360. [DOI] [PubMed] [Google Scholar]
- 47.Quinn DI, Aparicio A, Tsao-Wei DD, et al. Phase II study of eribulin (E7389) in patients (pts) with advanced urothelial Cancer (UC) – final report: a California Cancer Consortium-led NCI/CTEP-sponsored trial. J Clin Oncol. 2010;28(Suppl. 7) Abstract 4539. [Google Scholar]
- 48.Synold T, Tsao-Wei DD, Quinn DI, et al. Phase I and pharmacokinetic (PK) study of eribulin (E7389) in patients (pts) with renal dysfunction (RD) and advanced urothelial Cancer (UC): a California Cancer Consortium Trial. J Clin Oncol. 2010;28(Suppl. 7) Abstract 2527. [Google Scholar]
- 49.Pao W, Miller VA. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung Cancer: current knowledge and future directions. J Clin Oncol. 2005;23(11):2556–2568. doi: 10.1200/JCO.2005.07.799. [DOI] [PubMed] [Google Scholar]
- 50.Mok TS, Ramalingam SS. Maintenance therapy in nonsmall-cell lung Cancer: a new treatment paradigm. Cancer. 2009;115(22):5143–5154. doi: 10.1002/cncr.24563. [DOI] [PubMed] [Google Scholar]
- 51.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 52.Hudis CA. Trastuzumab – mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 53.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal Cancer. N Engl J Med. 2004;351(4):337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 54.Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal Cancer. N Engl J Med. 2007;357(20):2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 55.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal. Cancer N Engl J Med. 2009;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 56.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal. Cancer N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 57.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal Cancer. J Clin Oncol. 2008;26(10):1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 58.Comen EA, Robson M. Inhibition of poly(ADP)-ribose polymerase as a therapeutic strategy for breast Cancer. Oncology (Williston Park) 2010;24(1):55–62. [PubMed] [Google Scholar]
- 59.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast Cancer. N Engl J Med. 2007;357(26):2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 60.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast Cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 61.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 62.Lonn U, Lonn S, Friberg S, et al. Prognostic value of amplification of c-erb-B2 in bladder carcinoma. Clin Cancer Res. 1995;1(10):1189–1194. [PubMed] [Google Scholar]
- 63.Jimenez RE, Hussain M, Bianco FJ, Jr, et al. Her-2/neu overexpression in muscle-invasive urothelial carcinoma of the bladder: prognostic significance and comparative analysis in primary and metastatic tumors. Clin Cancer Res. 2001;7(8):2440–2447. [PubMed] [Google Scholar]
- 64.Bose R, Molina H, Patterson AS, et al. Phosphoproteomic analysis of Her2/neu signaling and inhibition. Proc Natl Acad Sci USA. 2006;103(26):9773–9778. doi: 10.1073/pnas.0603948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lae M, Couturier J, Oudard S, et al. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder Cancer with a standardized methodology: results in 1005 patients. Ann Oncol. 2010;21(4):815–819. doi: 10.1093/annonc/mdp488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansel DE, Swain E, Dreicer R, Tubbs RR. HER2 overexpression and amplification in urothelial carcinoma of the bladder is associated with MYC coamplification in a subset of cases Am. J Clin Pathol. 2008;130(2):274–281. doi: 10.1309/41VLTFX3YPP1HF6F. [DOI] [PubMed] [Google Scholar]
- 67.Buzdar AU. Role of biologic therapy and chemotherapy in hormone receptor- and HER2-positive breast Cancer. Ann Oncol. 2009;20(6):993–999. doi: 10.1093/annonc/mdn739. [DOI] [PubMed] [Google Scholar]
- 68.Hussain MH, MacVicar GR, Petrylak DP, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter Phase II National Cancer Institute trial. J Clin Oncol. 2007;25(16):2218–2224. doi: 10.1200/JCO.2006.08.0994. [DOI] [PubMed] [Google Scholar]
- 69.Carney WP, Neumann R, Lipton A, et al. Potential clinical utility of serum HER-2/neu oncoprotein concentrations in patients with breast Cancer. Clin Chem. 2003;49(10):1579–1598. doi: 10.1373/49.10.1579. [DOI] [PubMed] [Google Scholar]
- 70.Zhau HE, Zhang X, von Eschenbach AC, et al. Amplification and expression of the c-erb B-2/neu proto-oncogene in human bladder Cancer. Mol Carcinog. 1990;3(5):254–257. doi: 10.1002/mc.2940030503. [DOI] [PubMed] [Google Scholar]
- 71.Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal Cancer. J Clin Oncol. 2009;27(5):663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 72.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 73.Inoue K, Slaton JW, Perrotte P, et al. Paclitaxel enhances the effects of the anti-epidermal growth factor receptor monoclonal antibody ImClone C225 in mice with metastatic human bladder transitional cell carcinoma. Clin Cancer Res. 2000;6(12):4874–4884. [PubMed] [Google Scholar]
- 74.Perrotte P, Matsumoto T, Inoue K, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999;5(2):257–265. [PubMed] [Google Scholar]
- 75.Reck M. Gefitinib in the treatment of advanced non-small-cell lung Cancer. Expert Rev AntiCancer Ther. 2009;9(4):401–412. doi: 10.1586/era.09.1. [DOI] [PubMed] [Google Scholar]
- 76.Dominguez-Escrig JL, Kelly JD, Neal DE, King SM, Davies BR. Evaluation of the therapeutic potential of the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib in preclinical models of bladder Cancer. Clin Cancer Res. 2004;10(14):4874–4884. doi: 10.1158/1078-0432.CCR-04-0034. [DOI] [PubMed] [Google Scholar]
- 77.Philips GK, Halabi S, Sanford BL, Bajorin D, Small EJ. A Phase II trial of cisplatin (C), gemcitabine (G) and gefitinib for advanced urothelial tract carcinoma: results of Cancer and Leukemia Group B (CALGB) 90102. Ann Oncol. 2009;20(6):1074–1079. doi: 10.1093/annonc/mdn749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jacobs MA, Wotkowicz C, Baumgart ED, et al. Epidermal growth factor receptor status and the response of bladder carcinoma cells to erlotinib. J Urol. 2007;178(4 Pt 1):1510–1514. doi: 10.1016/j.juro.2007.05.113. [DOI] [PubMed] [Google Scholar]
- 79.McHugh LA, Kriajevska M, Mellon JK, Griffths TR. Combined treatment of bladder Cancer cell lines with lapatinib and varying chemotherapy regimens – evidence of schedule-dependent synergy. Urology. 2007;69(2):390–394. doi: 10.1016/j.urology.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 80.Wulfng C, Machiels JP, Richel DJ, et al. A single-arm, multicenter, open-label Phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer. 2009;115(13):2881–2890. doi: 10.1002/cncr.24337. [DOI] [PubMed] [Google Scholar]
- 81.Bernardini S, Fauconnet S, Chabannes E, et al. Serum levels of vascular endothelial growth factor as a prognostic factor in bladder Cancer. J Urol. 2001;166(4):1275–1279. [PubMed] [Google Scholar]
- 82•.Bochner BH, Cote RJ, Weidner N, et al. Angiogenesis in bladder Cancer: relationship between microvessel density and tumor prognosis. J Natl Cancer Inst. 1995;87(21):1603–1612. doi: 10.1093/jnci/87.21.1603. Describes the establishment of tumor angiogenesis, as measured by microvessel density, as an independent prognostic factor in patients with muscle-invasive bladder Cancer. [DOI] [PubMed] [Google Scholar]
- 83.Inoue K, Slaton JW, Karashima T, et al. The prognostic value of angiogenesis factor expression for predicting recurrence and metastasis of bladder Cancer after neoadjuvant chemotherapy and radical cystectomy. Clin Cancer Res. 2000;6(12):4866–4873. [PubMed] [Google Scholar]
- 84.Davies JM, Goldberg RM. First-line therapeutic strategies in metastatic colorectal Cancer. Oncology (Williston Park) 2008;22(13):1470–1479. [PubMed] [Google Scholar]
- 85.Traina TA. Bevacizumab in the treatment of metastatic breast Cancer. Oncology (Williston Park) 2009;23(4):327–332. [PubMed] [Google Scholar]
- 86.Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung Cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 87.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon α-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind Phase III trial. Lancet. 2007;370(9605):2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 88.Hahn N, Stadler WM, Zon RT, et al. A multicenter Phase II study of cisplatin (C), gemcitabine (G), and bevacizumab (B) as first-line chemotherapy for metastatic urothelial carcinoma (UC): Hoosier Oncology Group GU-0475. J Clin Oncol. 2009;27(Suppl. 15) doi: 10.1200/JCO.2010.31.6067. Abstract 5018. [DOI] [PubMed] [Google Scholar]
- 89.Elfiky AA, Rosenberg JE. Targeting angiogenesis in bladder Cancer. Curr Oncol Rep. 2009;11(3):244–249. doi: 10.1007/s11912-009-0034-2. [DOI] [PubMed] [Google Scholar]
- 90.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 91••.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon α in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. Randomized Phase III study comparing sunitinib with IFN-α in metastatic renal cell carcinoma. This trial established the improved RR and PFS with sunitinib. [DOI] [PubMed] [Google Scholar]
- 92.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized Phase III trial. J Clin Oncol. 2010;28(6):1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 93.Sridhar SS, Winquist E, Eisen A, et al. A Phase II trial of sorafenib in first-line metastatic urothelial Cancer: a study of the PMH Phase II Consortium. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9408-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 94.Dreicer R, Li H, Stein M, et al. Phase 2 trial of sorafenib in patients with advanced urothelial Cancer: a trial of the Eastern Cooperative Oncology Group. Cancer. 2009;115(18):4090–4095. doi: 10.1002/cncr.24467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sonpavde G, Jian W, Liu H, et al. Sunitinib malate is active against human urothelial carcinoma and enhances the activity of cisplatin in a preclinical model. Urol Oncol. 2009;27(4):391–399. doi: 10.1016/j.urolonc.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 96••.Gallagher DJ, Milowsky MI, Gerst SR, et al. Phase II study of sunitinib in patients with metastatic urothelial Cancer. J Clin Oncol. 2010;28(8):1373–1379. doi: 10.1200/JCO.2009.25.3922. Demonstrates activity for the novel anti-angiogenic agent, sunitinib, in patients with metastatic UC, thereby establishing the VEGF axis as a viable target in this disease. [DOI] [PubMed] [Google Scholar]
- 97.Bradley DA, Dunn R, Nanus D, et al. Randomized, double-blind, placebo-controlled Phase II trial of maintenance sunitinib versus placebo after chemotherapy for patients with advanced urothelial carcinoma: scientific rationale and study design. Clin Genitourin Cancer. 2007;5(7):460–463. doi: 10.3816/CGC.2007.n.037. [DOI] [PubMed] [Google Scholar]
- 98.Bukowski RM, Yasothan U, Kirkpatrick P. Pazopanib. Nat Rev Drug Discov. 2010;9(1):17–18. doi: 10.1038/nrd3073. [DOI] [PubMed] [Google Scholar]
- 99.Wedge SR, Ogilvie DJ, Dukes M, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002;62(16):4645–4655. [PubMed] [Google Scholar]
- 100.Ciardiello F, Caputo R, Damiano V, et al. Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clin Cancer Res. 2003;9(4):1546–1556. [PubMed] [Google Scholar]
- 101.Matsumori Y, Yano S, Goto H, et al. ZD6474, an inhibitor of vascular endothelial growth factor receptor tyrosine kinase, inhibits growth of experimental lung metastasis and production of malignant pleural effusions in a non-small cell lung Cancer model. Oncol Res. 2006;16(1):15–26. doi: 10.3727/000000006783981260. [DOI] [PubMed] [Google Scholar]
- 102.Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small cell lung Cancer (NSCLC): a randomized, double-blind Phase III trial (ZODIAC) J Clin Oncol. 2009;27(Suppl. 18) doi: 10.1016/S1470-2045(10)70132-7. Abstract CRA8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McKeage MJ, Baguley BC. Disrupting established tumor blood vessels: an emerging therapeutic strategy for Cancer. Cancer. 2010;116(8):1859–1871. doi: 10.1002/cncr.24975. [DOI] [PubMed] [Google Scholar]
- 104.Cao Y. Molecular mechanisms and therapeutic development of angiogenesis inhibitors. Adv Cancer Res. 2008;100:113–131. doi: 10.1016/S0065-230X(08)00004-3. [DOI] [PubMed] [Google Scholar]
- 105.Ching LM, Cao Z, Kieda C, et al. Induction of endothelial cell apoptosis by the antivascular agent 5,6-dimethylxanthenone-4-acetic acid. Br J Cancer. 2002;86(12):1937–1942. doi: 10.1038/sj.bjc.6600368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Siim BG, Lee AE, Shalal-Zwain S, et al. Marked potentiation of the antitumour activity of chemotherapeutic drugs by the antivascular agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) Cancer Chemother Pharmacol. 2003;51(1):43–52. doi: 10.1007/s00280-002-0529-0. [DOI] [PubMed] [Google Scholar]
- 107.Green C, Griffths-Johnson D, Dunmore K, et al. Marked potentiation of the in vivo antitumor activity of docetaxel in a human prostate Cancer xenograft by the vascular targeting agent 5,6 dimethyl xanthenone acetic acid, DMXAA. Proc Am Assoc Cancer Res. 2005;46 Abstract 2990. [Google Scholar]
- 108.Canes D, Chiang GJ, Billmeyer BR, et al. Histone deacetylase inhibitors upregulate plakoglobin expression in bladder carcinoma cells and display antineoplastic activity in vitro and in vivo. Int J Cancer. 2005;113(5):841–848. doi: 10.1002/ijc.20634. [DOI] [PubMed] [Google Scholar]
- 109.Cheung EM, Quinn DI, Tsao-Wei DD, et al. Phase II study of vorinostat (suberoylanilide hydroxamic acid, SAHA) in patients with advanced transitional cell urothelial Cancer (TCC) after platinum-based therapy – California Cancer Consortium/University of Pittsburgh NCI/CTEP-sponsored trial. J Clin Oncol. 2008;26(Suppl. 15) Abstract 16058. [Google Scholar]
- 110.Shang D, Liu Y, Matsui Y, et al. Demethylating agent 5-aza-2′-deoxycytidine enhances susceptibility of bladder transitional cell carcinoma to cisplatin. Urology. 2008;71(6):1220–1225. doi: 10.1016/j.urology.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 111.Kamat AM, Karashima T, Davis DW, et al. The proteasome inhibitor bortezomib synergizes with gemcitabine to block the growth of human 253JB-V bladder tumors in vivo. Mol Cancer Ther. 2004;3(3):279–290. [PubMed] [Google Scholar]
- 112.Lashinger LM, Zhu K, Williams SA, et al. Bortezomib abolishes tumor necrosis factor-related apoptosis-inducing ligand resistance via a p21-dependent mechanism in human bladder and prostate Cancer cells. Cancer Res. 2005;65(11):4902–4908. doi: 10.1158/0008-5472.CAN-04-3701. [DOI] [PubMed] [Google Scholar]
- 113.Gomez-Abuin G, Winquist E, Stadler WM, et al. A Phase II study of PS-341 (bortezomib) in advanced or metastatic urothelial Cancer A trial of the Princess Margaret Hospital and University of Chicago Phase II consortia. Invest New Drugs. 2007;25(2):181–185. doi: 10.1007/s10637-006-9009-4. [DOI] [PubMed] [Google Scholar]
- 114.Rosenberg JE, Halabi S, Sanford BL, et al. Phase II study of bortezomib in patients with previously treated advanced urothelial tract transitional cell carcinoma: CALGB 90207. Ann Oncol. 2008;19(5):946–950. doi: 10.1093/annonc/mdm600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Billerey C, Chopin D, Aubriot-Lorton MH, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158(6):1955–1959. doi: 10.1016/S0002-9440(10)64665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Knowles MA. Molecular pathogenesis of bladder Cancer. Int J Clin Oncol. 2008;13(4):287–297. doi: 10.1007/s10147-008-0812-0. [DOI] [PubMed] [Google Scholar]
- 117.Bakkar AA, Wallerand H, Radvanyi F, et al. FGFR3 and TP53 gene mutations define two distinct pathways in urothelial cell carcinoma of the bladder. Cancer Res. 2003;63(23):8108–8112. [PubMed] [Google Scholar]
- 118••.Platt FM, Hurst CD, Taylor CF, et al. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder Cancer. Clin Cancer Res. 2009;15(19):6008–6017. doi: 10.1158/1078-0432.CCR-09-0898. Comprehensive analysis of the PI3K–Akt pathway in bladder Cancer, incorporating mutation data, copy number alterations, and protein expression derived from bladder tumors and representative cell lines. [DOI] [PubMed] [Google Scholar]
- 119.Lee SH, Lopes de Menezes D, Vora J, et al. In vivo target modulation and biological activity of CHIR-258, a multitargeted growth factor receptor kinase inhibitor, in colon Cancer models. Clin Cancer Res. 2005;11(10):3633–3641. doi: 10.1158/1078-0432.CCR-04-2129. [DOI] [PubMed] [Google Scholar]
- 120.Sarker D, Molife R, Evans TR, et al. A Phase I pharmacokinetic and pharmacodynamic study of TKI258, an oral, multitargeted receptor tyrosine kinase inhibitor in patients with advanced solid tumors. Clin Cancer Res. 2008;14(7):2075–2081. doi: 10.1158/1078-0432.CCR-07-1466. [DOI] [PubMed] [Google Scholar]
- 121.Yuan TL, Cantley LC. PI3K pathway alterations in Cancer: variations on a theme. Oncogene. 2008;27(41):5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 123.Hay N. The Akt–mTOR tango and its relevance to Cancer. Cancer Cell. 2005;8(3):179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 124.Knowles MA, Platt FM, Ross RL, Hurst CD. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder Cancer. Cancer Metastasis Rev. 2009;28(3–4):305–316. doi: 10.1007/s10555-009-9198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jebar AH, Hurst CD, Tomlinson DC, et al. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene. 2005;24(33):5218–5225. doi: 10.1038/sj.onc.1208705. [DOI] [PubMed] [Google Scholar]
- 126.Engelman JA. Targeting PI3K signalling in Cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 127.She QB, Chandarlapaty S, Ye Q, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS ONE. 2008;3(8):e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of Cancer cells with activating PI3K mutations. Cancer Res. 2008;68(19):8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 129.Gibbons JJ, Abraham RT, Yu K. Mammalian target of rapamycin: discovery of rapamycin reveals a signaling pathway important for normal and Cancer cell growth. Semin Oncol. 2009;36(Suppl. 3):S3–S17. doi: 10.1053/j.seminoncol.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 130.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled Phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 131.Tickoo SK, Milowsky MI, Dhar N, et al. Hypoxia inducible factor (HIF) and mammalian target of rapamycin (mTOR) pathway markers in urothelial carcinoma of the bladder: possible therapeutic implications. BJU Int. 2010 doi: 10.1111/j.1464-410X.2010.09517.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Website
- 201.Press release AP. ATTRACT-1 Phase III trial of ASA404 halted following interim analysis. www.antisoma.com/