Abstract
Background
With ever increasing pressure to reduce costs and increase quality, nurses are faced with the challenge of producing evidence that their interventions and care provide value. Cost effectiveness analysis (CEA) is a tool that can be used to provide this evidence by comparative evaluation of the costs and consequences of two or more alternatives.
Objectives
The aim of this article is to introduce the essential components of CEA to nurses and nurse researchers with the protocol of a recently funded cluster randomized controlled trial as an example.
Methods
This article provides: (a) a description of the main concepts and key steps in CEA, and (b) a summary of the background and objectives of a CEA designed to evaluate a nursing led pain and symptom management intervention in rural communities compared to current usual care.
Discussion
As the example highlights, incorporating CEA into nursing research studies is feasible. The burden of the additional data collection required is off-set by quantitative evidence of the given intervention's cost and impact using humanistic and economic outcomes. At a time when US health care is moving toward accountable care, the information provided by CEA will be an important additional component of the evidence produced by nursing research.
Keywords: cost effectiveness, cluster randomized clinical trial, pain management, rural health
The rising cost of U.S. health care has been a political issue for almost 40 years (McMahon & Chopra, 2012). Many attempts have been made to mitigate health care costs while ensuring that the quality of patient care does not suffer in the process. Over the last 40 years managed care, as well as hospital and physician payment reforms, has come and gone without success (Berenson & Rich, 2010). The Patient Protection and Affordable Care Act (P-PACA) (2010) represents the latest national effort to improve quality and reduce health care costs.
A key component of P-P ACA is the development of health care systems innovations, such as Accountable Care Organizations (ACOs), that aim to meet the P-PACA goal of ensuring accessible, high-quality, and affordable health care for all Americans (U.S. Department of Health and Human Services, 2011). ACOs move beyond the expansion of health care insurance coverage to also include shift in payment incentives from volume (as seen in a fee-for-service model) to quality and outcomes (Fisher, Bynum, & Skinner, 2009; Goroll & Schoenbaum, 2012; Sommers & Bindman, 2012).
This shift has created an increasingly competitive environment. Nurses, as health care providers, leaders, and advocates of high-quality, patient-centered, and cost effective health care (Hart, 2012), will need to provide evidence of the value they provide. Cost effectiveness analysis (CEA) is a tool that can provide this evidence by evaluating both the costs and effectiveness of nursing interventions. While evidence of clinical effectiveness is essential for health care decision makers, the results of well-designed cost effectiveness studies provides important additional information on costs and effectiveness for policy makers, payers, and consumers. The aim of this article is to introduce the essential components of CEA to nurses and nurse researchers. The protocol of a recently funded cluster randomized controlled trial “Symptom Management in Rural Communities” will be used as an example.
Methods
Main Concepts and Key Steps
Scarcity of resources creates the need to make choices about how available resources will be used. Not all needs or wants can be met. Some interventions are not feasible, and the resultant benefits unattainable, because their costs exceed available resources (Rhodes, Battin, & Silvers, 2012). It is thus important to maximize return from the resources that are available.
CEA is a tool used for comparative analysis of different ways of using scarce health care resources. This involves comparing current “usual care” to one or more alternatives in terms of both the costs and effectiveness involved. CEA provides a systematic approach for balancing resource implications (cost) against results (effectiveness) of alternatives and is preferable to other approaches such as “gut feelings”, “what we have always done”, or “educated guesses” (Drummond, Sculpher, Torrance, O'Brien, & Stoddart, 2005). The results of CEA provide decision makers with information on costs, effectiveness, and the relationship between the two (i.e., the value attained).
Conducting a CEA is a complex and multifaceted undertaking. The process can be broken into five key steps (Bensink, Scuffham,& Smith 2012): (a) formulate the research question to be answered, (b) define and measure the resources used and consumed, and then assign costs, (c) define and measure effectiveness, (d) complete incremental analysis including estimates of uncertainty, and (e) present and interpret results.
Formulate the research question
This first step is critical because it determines the foundation of the methods to be used in the analysis and the resulting evidence provided. The patient, intervention, comparator, and outcome (PICO) structure (Centre for Evidence-Based Medicine, 2009), with important information relating specifically to economic evaluation added to the clinical research question, provides a single comprehensive statement that captures the key components of the analysis (Bensink et al., 2012). Economic information added to the clinical research question includes: (a) the economic perspective of the analysis, (b) the economic decision maker that the evidence is directed toward, and (c) the explicit focus on cost effectiveness versus clinical effectiveness as the outcome of interest.
Define and measure resources used/consumed and assign costs
Including the economic perspective and information on the economic decision maker in the research question has important implications for the scope of the data collection required. As recommended by the United States Preventive Services Task Force (USPSTF) Panel on Cost Effectiveness in Health and Medicine (Gold, Siegel, Russell, & Weinstein, 1996), a broad societal perspective should be used to capture all of the anticipated and unanticipated impacts of the intervention(s) under evaluation. The USPSTF outlines four specific areas: (a) the health care resources used and/or consumed, including those required to provide the intervention; (b) the non-health care resources used and/or consumed by patients to receive care; (c) patient time to access care, and (d) the resources provided by family caregivers. Measuring the use of these resources should be undertaken using a combination of patient interviews, chart reviews, tracking logs and potentially hospital bills or claims data (depending on availability) (Ramsey et al., 2005).
Once the relevant resources have been identified, they need to be valued based on credible sources. For health care resources used and/or consumed, credible sources in the United States include the Healthcare Cost and Utilization Project (Steiner, Elixhauser, & Schnaier, 2002) and the Diagnosis Related Group Weights and Physician Fee Schedules (Centers for Medicare and Medicaid Services, 2012). For context specific resources, additional information from secondary hospital and clinic financial systems can be used where available (Ramsey et al., 2005). For items like patient and caregiver time, age- and sex-adjusted national wage rates can be used (Weinstein, Siegel, Gold, Kamlet, & Russell, 1996).
It is essential to provide the decision maker specified in the research question with information relevant to their specific decision-making context, measuring the disaggregate components of the resources involved, so results from alternate perspectives, such as health care system and patient/family perspectives, can be presented in the analysis (Drummond et al., 2005).
Define and measure effectiveness
Because CEA provides evidence to decision makers who are most likely considering the impact of a wide range of alternative uses of scarce health care resources, CEA requires looking at effectiveness in a way that can be compared across many different diseases, patient groups, interventions, therapies, and approaches. Disease specific measures cannot be used to compare the effectiveness of all possible uses of the resources available to decision makers. Although not a perfect measure (Nord, Daniels, & Kamlet, 2009), quality-adjusted life years (QALYs) is the currently accepted measure of effectiveness for CEAs (Drummond, et al., 2005; Gold, et al., 1996; Weinstein, Torrance, & McGuire, 2009). The QALYs variable combines both quality and quantity of life information into one common measure that can be used to compare very different interventions.
While there are a number of ways to obtain information for the calculation of QALYs, the use of multiattribute health status classification systems with preference scores has gained increasing popularity with researchers over time. Ease of use and the minimization of participant burden are some of the key factors in this trend. For example, the Health Utilities Index Version 3 (HUI 3) asks patients to rate their current health status across eight attributes: vision, hearing, speech, ambulation, dexterity, emotion, cognition, and pain (Feeny, Furlong, Boyle, & Torrance, 1995; Furlong, Feeny, Torrance, & Barr, 2001). Responses are then valued using a scoring and health state preferences algorithm. The resulting utility scores reflect the quality of life of patients on a scale from 0 = dead to 1 = perfect health and can be used to calculate QALYs.
Complete incremental analysis including estimates of uncertainty
After the collection and valuation of cost and effectiveness data, the next step in a CEA is analysis. The arithmetic mean cost for patients in intervention and control groups is estimated followed by an incremental analysis of the arithmetic mean difference in cost between groups. From a societal perspective, this provides a summary estimate of the average net impact of the intervention on total per patient cost. An indication of the uncertainty surrounding these estimates is provided by reporting means with 95% confidence intervals. A p-value should also be reported for the incremental analysis in trial-based evaluations (Glick, Doshi, Sonnad, & Polsky, 2007).
While other statistical measures can be used to characterize these estimates, the arithmetic mean is the important statistic in CEA. This is due to CEA's unique budgetary and societal perspectives (Thompson & Barber, 2000). This can present analytic challenges as cost data are generally skewed with a long right tail. Guidance recommends completing simple univariate analysis first (e.g., independent samples t-tests when a new treatment is compared with usual care), followed by multivariable analysis using techniques such as the generalized linear model if the distributional properties of the data are an issue (Glick et al., 2007). Similarly, information from the QALY information collected from patients is used to estimate the arithmetic mean for patients in each group along with the difference between means and the probability associated with this difference compared to a hypothesis of no difference.
Estimates of the arithmetic mean differences in cost (Δc), relative to the arithmetic mean difference in QALY effectiveness (Δe), are then compared to produce an incremental cost effectiveness ratio (ICER) such that ICER = Δc / Δe. The ICER provides information on the value provided by the new intervention compared to current usual care. The metric of value is the average cost needed to produce an average gain of one quality-adjusted life year ($ / QALY).
As with the analysis of cost and effectiveness, the uncertainty associated with the ICER (the joint distribution of uncertainty surrounding cost and QALY estimates) is also included using a 95% confidence interval. A number of analytic techniques that can be used, including the commonly implemented bootstrap percentile method (Briggs, Wonderling, & Mooney, 1997; Polsky, Glick, Willke, & Schulman, 1997).
Present and interpret results
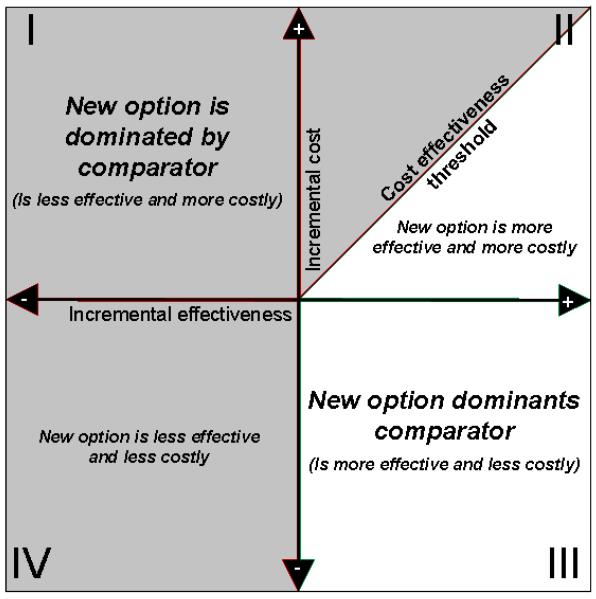
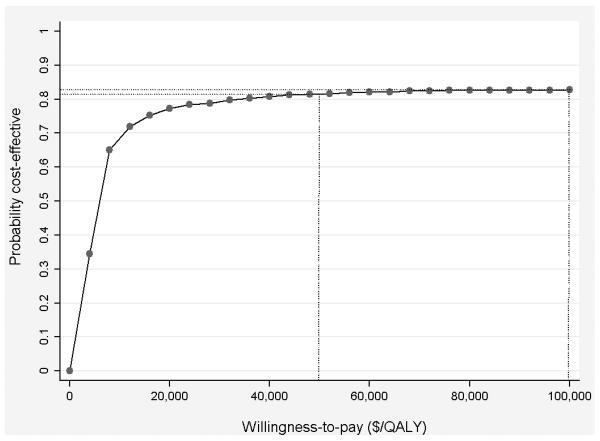
Results are presented on the cost effectiveness plane (Black, 1990) to provide a graphical summary of the joint distribution of costs and effectiveness along with associated uncertainty in estimates (Figure 1). Uncertainty in ICER results should also be characterized using willingness-to-pay (WTP) thresholds and cost effectiveness acceptability curves (CEACs) (Fenwick & Byford, 2005). This approach uses a pre-defined definition of value, the WTP threshold (e.g., $50,000 or $100,000 per QALY), to guide decision making (Figure 2). Alternatives that cost less than the threshold are considered cost effective; those over the threshold are not. A CEAC provides information on the probability that the alternative under evaluation is cost effective given the underlying uncertainty in ICER estimates.
Figure 1.
The four quadrants of the cost effectiveness plane. The four quadrants of the cost effectiveness plane provide information on the joint distribution of costs and effects. Quadrant III results show a new option that should be adopted as it is less costly and more effective than usual care. Quadrant II results below the cost effectiveness threshold should be adopted; those above should not as they exceed the predefined threshold (e.g., $50,000 or $100,000 per QALY). Results in quadrant IV could theoretically have a similar threshold if there is a willingness to accept decreased effectiveness to reduce cost, but in practice, a less effective option is not adopted. Adapted from “The CE Plane: A graphic Representation of Cost-Effectiveness” by W.C. Black, 1990, Medical Decision Making, 10, 212–214. Copyright 1990, Sage Publications. Used with permission.
Figure 2.
Cost effectiveness acceptability curve. This simulated cost effectiveness acceptability curve shows that the probability that the new option is cost-effective, compared to the comparator, is .819 at a willingness-to-pay threshold of $50,000/QALY. The probability at $100,000/QALY is slightly higher, at .832.
Example CEA: Symptom Management Study
The following illustrates the application of these methodological aspects of CEA. The “Symptom Management in Rural Communities” cluster randomized trial currently underway is used as an example.
Study background and objectives
The Office of Rural Health Policy accepts all non-metro counties as rural areas in the United States (Health Resources and Services Administration, 2013). Yet rural residents are more likely than their urban counterparts to be: older; in poorer overall health; suffering from more chronic or serious illnesses and disabilities; uninsured or under-insured; and living in poverty (Rosenthal & Fox, 2000). Although telehealth (the use of information and communication technologies to deliver health care at a distance) is an emerging method of health care delivery that has been found useful and effective in many clinical settings and specialties (Hersh, Helfand et al., 2002; Hersh, Hickam et al. 2006), its effectiveness and the associated resource implications for symptom management have yet to be explored. Thus, the basis of the study described here is the evaluation of the effectiveness of a telehealth-enhanced symptom-management intervention among rural health care providers and the patients they care for. Correspondingly, the study is designed to answer the question: for rural-dwelling patients, does a telehealth-enhanced symptom-management intervention provide better pain management than current usual care?
Study design
The study is designed as a cluster randomized trial with a wait-list control group and is conducted with rural providers in the Washington, Wyoming, Alaska, Montana, and Idaho (WWAMI) region. (The study is approved by the institutional review board of the University of Washington.) Eligible providers in each site are those responsible for direct patient care including: physicians, physician assistants, and nurse practitioners. Consenting providers are asked to identify patients under their care who are being seen for chronic pain. Patients who agree to participate in the study are then contacted by phone and provided a description of the study. Two additional inclusion criteria are assessed at this time: those patients score a 3 or higher on a 10-point pain scale and are functionally fluent in English. Eligible patients are then asked to consent to participate in the study before being assigned to the same group as their provider.
Providers randomized to the “integrated symptom management intervention group”, have access to weekly videoconferences with other community providers and university-based pain and symptom management experts to manage cases, engage in evidence-based practice activities, and receive peer support. Providers randomized to the wait-list control group, provide usual care to patients at their specific site (i.e., without evidence-based videoconferencing or peer support activities) until week 12, when they are also given access to the intervention.
The primary outcome measure for the study is pain severity assessed using PainTracker, a web-based patient-reported pain outcomes measurement tool. PainTracker is also used to collect secondary outcome measures including anxiety and depression (Spitzer, Kroenke, & Williams, 1999; Spitzer, Kroenke, Williams, & Lowe, 2006), overall quality-of-life (HUI3), as well as fatigue, dyspnea, and constipation. To account for nesting of patients within providers, and providers within study sites, the impact of the intervention on the primary outcome of patient-reported pain management outcomes will be analyzed using a hierarchical linear model (HLM) (Raudenbush, Bryk, Cheong, & Congdon, 2004). The results of this analysis will provide an answer to the study's primary research question.
Integrated cost effectiveness analysis
Given the resource constraints experienced by rural communities (Baldwin et al., 2006; Jukkala, Henly, & Lindeke, 2008), the completion of an economic evaluation alongside the primary comparative effectiveness study is particularly compelling. Aligned with the primary study's research question, the economic research question is: from a societal perspective, should rural health care organizations caring for adult patients, invest in telehealth-enhanced symptom management as a cost effective alternative to current usual care?
A societal perspective is taken for the CEA. This includes the resources that will be used/consumed in the four specific areas recommended by the USPSTF (Table 1). The study is also placed in a particular decision-making context, that of the rural health care organizations responsible for the care of adult patients.
Table 1.
Cost components to be included in a societal perspective cost effectiveness analysis
| Cost Components | Symptom Management in Rural Communities CEA |
|---|---|
| Healthcare Resources Perspective: Rural Provider Organization/Payer |
Resources required to provide: Integrated Symptom Management Intervention
|
| Nonhealthcare Resources Perspective: Patient/Family |
Out-of-pockets expenses for:
|
| Informal Caregiver Time Perspective: Patient/Family |
Additional time spent:
|
| Patient Time Perspective: Patient/Family |
Additional time spent:
|
| Perspective: Societal | Total |
For effectiveness, the HUI3 was selected due to its: (a) credibility as an established instrument with demonstrated feasibility, reliability, validity, and responsiveness (Horsman, Furlong, Feeney, & Torrance, 2003); (b) coverage of the health attributes likely to be important to the patient population under study (ambulation, emotion, cognition, and pain); and (c) prior use in similar patient populations (Franks, Hanmer, & Fryback, 2006; Lubetkin & Gold, 2002; Räsänen et al., 2006).
As an adaptation to the multivariate techniques recommended, analysis of differences between cost and effectiveness will use the same multivariate HLM technique being used for the primary study question to account for both the correlation between costs and outcomes and the potential dependence of these data on clustering under the enrolled clinician (Gomes, Grieve, Nixon, & Edmunds, 2012). Numeric results will be presented aggregated and disaggregated from societal, rural health care organization, and patient/family perspectives. Overall results of cost effectiveness will be presented using the cost effectiveness plane and as a cost effectiveness acceptability curve.
Discussion
Incorporating an analysis of cost effectiveness alongside a comparative effectiveness study adds specific data collection requirements and analytic activities to the primary study. This includes the collection, valuation, and analysis of comprehensive information on the resources used from the recommended societal perspective, as well as information from patients that allows the recommended measure of effectiveness, the QALY, to be calculated. Although these additions, as presented here, may seem relatively straightforward, there are many important decisions to be made and many different facets of any analysis that need to be considered. Including a health economist to study personnel from the beginning and maintaining their involvement throughout a study is essential.
The inclusion of CEA as part of a comparative effectiveness evaluation provides a number of benefits. These include: comprehensive information on the cost of implementing an intervention, the resulting impact of this investment on the total cost of patient care, the cost to patients, the cost to informal caregivers, the effectiveness of the intervention as a means of improving the overall health of the patient population under study, and the value provided by using the new alternative over the currently accepted usual care.
The plan to conduct a comprehensive economic evaluation of a telehealth-enhanced symptom-management intervention among rural patients, from the recommended societal perspective and the composite perspectives this involves, will be valuable to rural provider organizations, patients, their families, and the clinicians, including nurses who care for them. It will also provide important information to decision makers working for public and private health insurance plans especially when considerations of cost effectiveness are increasingly used in the private sector to make coverage and reimbursement policies (Malone, 2005; Neumann, 2004; Trice, Devine, Mistry, Moore, & Linton, 2009; Weart & Bauman, 2007).
Despite the value of this information, it is important to highlight that CEA evidence is not the only information that can or should be used for decision making. CEA provides additional evidence to supplement rather than replace the comparative-effectiveness evidence produced by analysis of primary clinical outcomes. Decision makers also consider other issues such as availability, access and equity. At a time when U.S. health care is moving toward accountable care, the information provided by CEA will be an important additional component of the evidence produced by nursing research.
Acknowledgments
This work was supported by a grant from the National Institutes of Health/National Institute of Nursing Research (R01 NR012450) and the University of Washington Palliative Care Center of Excellence.
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldwin LM, Patanian MM, Larson EH, Lishner DM, Mauksch LB, Katon WJ, Hart LG. Modeling the mental health workforce in Washington State: Using state licensing data to examine provider supply in rural and urban areas. Journal of Rural Health. 2006;22:50–58. doi: 10.1111/j.1748-0361.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Bensink ME, Scuffham PA, Smith AC. Health economics. In: Soyer HP, Binder M, Smith AC, Wurm EMT, editors. Telemedicine in dermatology. Springer; New York, NY: 2012. pp. 167–185. [Google Scholar]
- Berenson RA, Rich EC. U.S. approaches to physician payment: the deconstruction of primary care. Journal of General Internal Medicine. 2010;25:613–618. doi: 10.1007/s11606-010-1295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black WC. The CE plane: A graphic representation of cost effectiveness. Medical Decision Making. 1990;10:212–214. doi: 10.1177/0272989X9001000308. [DOI] [PubMed] [Google Scholar]
- Briggs A, Wonderling D, Mooney C. Pulling cost effectiveness analysis up by its bootstraps: A non-parametric approach to confidence interval estimation. Health Economics. 1997;6:327–340. doi: 10.1002/(sici)1099-1050(199707)6:4<327::aid-hec282>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services . Medicare Program; Payment Policies under the Physician Fee Schedule. Department of Health and Human Services; Baltimore, MD: 2012. [Google Scholar]
- Centre for Evidence-Based Medicine Asking focused questions. 2009 Retrieved from http://www.cebm.net/index.aspx?o=1036.
- Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford University Press; Oxford, UK: 2005. [Google Scholar]
- Feeny D, Furlong W, Boyle M, Torrance GW. Multi-attribute health status classification systems. Health Utilities Index. Pharmacoeconomics. 1995;7:490–502. doi: 10.2165/00019053-199507060-00004. [DOI] [PubMed] [Google Scholar]
- Fenwick E, Byford S. A guide to cost effectiveness acceptability curves [Editorial] British Journal of Psychiatry. 2005;187:106–108. doi: 10.1192/bjp.187.2.106. [DOI] [PubMed] [Google Scholar]
- Fisher ES, Bynum JP, Skinner JS. Slowing the growth of health care costs--lessons from regional variation. New England Journal of Medicine. 2009;360:849–852. doi: 10.1056/NEJMp0809794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks P, Hanmer J, Fryback DG. Relative disutilities of 47 risk factors and conditions assessed with seven preference-based health status measures in a national U.S. sample: Toward consistency in cost effectiveness analyses. Medical Care. 2006;44:478–485. doi: 10.1097/01.mlr.0000207464.61661.05. [DOI] [PubMed] [Google Scholar]
- Furlong WJ, Feeny DH, Torrance GW, Barr RD. The Health Utilities Index (HUI) system for assessing health-related quality of life in clinical studies. Annals of Medicine. 2001;33:375–384. doi: 10.3109/07853890109002092. [DOI] [PubMed] [Google Scholar]
- Glick H, Doshi JA, Sonnad SS, Polsky D. Economic evaluation in clinical trials. Oxford University Press; Oxford, UK: 2007. [Google Scholar]
- Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost effectiveness in health and medicine. Oxford University Press; Oxford, UK: 1996. [Google Scholar]
- Gomes M, Grieve R, Nixon R, Edmunds WJ. Statistical methods for cost effectiveness analyses that use data from cluster randomized trials: A systematic review and checklist for critical appraisal. Medical Decision Making. 2012;32:209–220. doi: 10.1177/0272989X11407341. [DOI] [PubMed] [Google Scholar]
- Goroll AH, Schoenbaum SC. Payment reform for primary care within the accountable care organization: A critical issue for health system reform. JAMA. 2012;308:577–578. doi: 10.1001/jama.2012.8696. [DOI] [PubMed] [Google Scholar]
- Hart MA. Accountable care organizations: the future of care delivery? American Journal of Nursing. 2012;112(2):23–26. doi: 10.1097/01.NAJ.0000411171.41245.0c. [DOI] [PubMed] [Google Scholar]
- Health Resources and Services Administration Rural Health. 2013 Retrieved April 24, 2013 from http://www.hrsa.gov/ruralhealth/policy/definition_of_rural.html.
- Hersh W, Helfand M, Wallace J, Kraemer D, Patterson P, Shapiro S, Greenlick M. A systematic review of the efficacy of telemedicine for making diagnostic and management decisions. Journal of Telemedicine and Telecare. 2002;8:197–209. doi: 10.1258/135763302320272167. [DOI] [PubMed] [Google Scholar]
- Hersh WR, Hickam DH, Severance SM, Dana TL, Pyle Krages K, Helfand M. Diagnosis, access and outcomes: Update of a systematic review of telemedicine services. Journal of Telemedicine and Telecare. 2006;12(Suppl 2):S3–S31. doi: 10.1258/135763306778393117. [DOI] [PubMed] [Google Scholar]
- Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): concepts, measurement properties and applications. Health and Quality of Life Outcomes. 2003;1(54) doi: 10.1186/1477-7525-1-54. doi:10.1186/1477-7525-1181-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukkala AM, Henly SJ, Lindeke LL. Rural perceptions of continuing professional education. Journal of Continuing Education in Nursing. 2008;39:555–563. doi: 10.3928/00220124-20081201-08. [DOI] [PubMed] [Google Scholar]
- Lubetkin EI, Gold MR. Comprehensibility of measures of health-related quality of life in minority and low-income patients. Journal of the National Medical Association. 2002;94:327–335. [PMC free article] [PubMed] [Google Scholar]
- Malone DC. The role of pharmacoeconomic modeling in evidence-based and value-based formulary guidelines. Journal of Managed Care Pharmacy. 2005;11(4 Suppl):S7–S10. doi: 10.18553/jmcp.2005.11.4.S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LF, Jr., Chopra V. Health care cost and value: The way forward. JAMA. 2012;307:671–672. doi: 10.1001/jama.2012.136. [DOI] [PubMed] [Google Scholar]
- Neumann PJ. Evidence-based and value-based formulary guidelines. Health Affairs. 2004;23:124–134. doi: 10.1377/hlthaff.23.1.124. [DOI] [PubMed] [Google Scholar]
- Nord E, Daniels N, Kamlet M. QALYs: Some challenges. Value in Health. 2009;12(Suppl 1):S10–S15. doi: 10.1111/j.1524-4733.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- Patient Protection and Affordable Care Act 2010. Pub. L. No. 111-148, 124 Stat. 119 (2010)
- Polsky D, Glick HA, Willke R, Schulman K. Confidence intervals for cost effectiveness ratios: A comparison of four methods. Health Economics. 1997;6:243–252. doi: 10.1002/(sici)1099-1050(199705)6:3<243::aid-hec269>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ramsey S, Wilke R, Briggs A, Brown R, Buxton M, Chawla A, Reed S. Good research practice for cost effectiveness analysis alongside clinical trials: The ISPOR RCT-CEA Task Force report. Value in Health. 2005;8:521–533. doi: 10.1111/j.1524-4733.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- Räsänen P, Roine E, Sintonen H, Semberg-Konttinen V, Ryynanen OP, Roine R. Use of quality-adjusted life years for the estimation of effectiveness of health care: A systematic literature review. International Journal of Technology Assessment in Health Care. 2006;22:235–241. doi: 10.1017/S0266462306051051. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, Cheong Y, Congdon RJ. HLM 6: Hierarchical linear and nonlinear modeling. Scientific Software; Lincolnwood, IL: 2004. [Google Scholar]
- Rhodes R, Battin MP, Silvers A, editors. Medicine and social justice: essays on the distribution of health care. 2nd ed. Oxford University Press; Oxford, UK: 2012. [Google Scholar]
- Rosenthal TC, Fox C. Access to health care for the rural elderly. JAMA. 2000;284:2034–2036. doi: 10.1001/jama.284.16.2034. [DOI] [PubMed] [Google Scholar]
- Sommers BD, Bindman AB. New physicians, the Affordable Care Act, and the changing practice of medicine. JAMA. 2012;307:1697–1698. doi: 10.1001/jama.2012.523. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary care evaluation of mental disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: An overview. Effective Clinical Practice. 2002;5:143–151. [PubMed] [Google Scholar]
- Thompson SG, Barber J. How should cost data in pragmatic randomised trials be analysed? British Medical Journal. 2000;320:1197–1200. doi: 10.1136/bmj.320.7243.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trice S, Devine J, Mistry H, Moore E, Linton A. Formulary management in the Department of Defense. Journal of Managed Care Pharmacy. 2009;15:133–146. doi: 10.18553/jmcp.2009.15.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Report to Congress: National Strategy for Quality Improvement in Health Care. Washington, DC: 2011. Retrieved from http://www.healthcare.gov/news/reports/quality03212011a.html. [Google Scholar]
- Weart W, Bauman GR. The case for a value-based formulary: striving for total lowest net cost. Managed Care Interface. 2007;20(4):42–47. [PubMed] [Google Scholar]
- Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- Weinstein MC, Torrance G, McGuire A. QALYs: The basics. Value Health. 2009;12(Suppl 1):S5–S9. doi: 10.1111/j.1524-4733.2009.00515.x. [DOI] [PubMed] [Google Scholar]