Fig. 5.
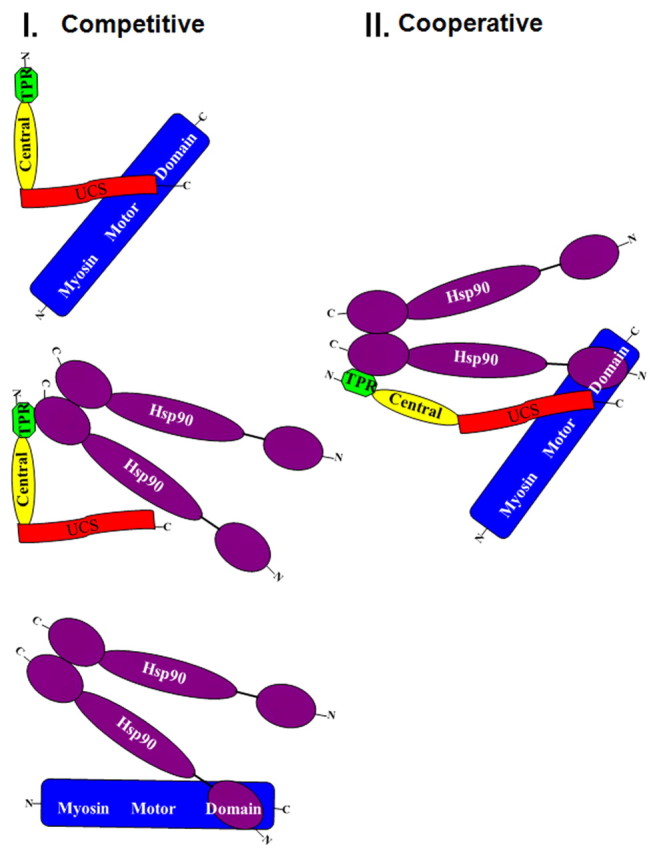
Alternative models of UNC-45, Hsp90 and myosin motor interactions. In model I, UNC-45 and Hsp90 compete for myosin motor binding; binding of Hsp90 to the TPR domain and of myosin to the UCS domain would be sterically incompatible with one another. In this model, only binary complexes of the combinations of the three proteins would be significant. In model II, UNC-45 and Hsp90 cooperate for myosin motor binding in a ternary complex. Model II would require a major conformational change from the crystallographically based L-shaped structure (Lee et al., 2011; Shi and Blobel, 2010) to accommodate binding, because the Hsp90 dimer and myosin motor region have molecular weights of 182,000 and 115,000, respectively, significantly greater than the 107,000 of UNC-45 (Barral et al., 1998; Barral et al., 2002). Hsp90 assumes the open structure because the in vitro binding experiments in Figs 4B and supplementary material Fig. S1 were performed in the absence of ATP. Previous experiments (A. H. H. and H. F. E., unpublished results) showed that myosin did not bind UNC-45 in the presence of ATP.
