Abstract
Gynecomastia is defined as an enlargement of the male breast. It is often benign, and can be the source of significant embarrassment and psychological distress. A general medical history and careful physical examination are essential to distinguish normal developmental variants from pathological causes. Treatment is geared toward the specific etiology when identified. In the majority of cases of pubertal gynecomastia, observation and reassurance are the mainstays of therapy as the condition usually resolves naturally. Pharmacological treatment and surgery are recommended only in selected cases.
Keywords: Gynecomastia, adolescent, surgical treatment, ultrasound-assisted liposuction
Gynecomastia, a glandular proliferation in the male breast, is a common clinical condition that may occur in males of all ages. “Gynecomastia” is derived from the Greek terms gynec (female) and mastos (breast) and was first coined by Galen in the second century AD. The condition may be an incidental finding on routine physical examination, or may present as new-onset palpable breast mass with or without mastalgia. It can be unilateral, bilateral, and/or asymmetrical. Pseudogynecomastia (fatty breasts) is commonly seen in obese males and differs from gynecomastia in that breast enlargement is due to increased fat deposition without glandular proliferation. Gynecomastia may cause significant embarrassment and psychological distress in affected males. In this article, the authors focus on pubertal gynecomastia and review the medical and surgical approaches to managing male adolescents with this condition.
Prevalence
During the life span, three phases of occurrence of gynecomastia have been observed, corresponding to times of hormonal changes. The first peak is found during the neonatal period, when an estimated 60 to 90% of infants develop transient palpable breast tissue because of the transplacental passage of estrogens.1,2 Gynecomastia in the newborn almost always regresses spontaneously and completely within the first year of life. The second peak occurs during puberty, when an incidence range of anywhere between 4 to 69% of palpable breast tissue and an increase in breast size has been reported.1 Gynecomastia may present as early as age 10, with a peak onset between the ages of 13 and 14 years, followed by a decline of incidence in late teenage years.1 By age 17, only 10% of boys are found to have persistent gynecomastia.3 In the largest cross-sectional study performed to date on gynecomastia in adolescents, the prevalence was found to be 4% in males aged 10 to 19 years.4 The third and last peak of occurrence is found later in life, with the highest prevalence among adults between the ages of 50 and 80 years.5,6 When comparing results of prevalence studies, it is important to note which criteria investigators have used to define gynecomastia. The diagnostic criterion has been defined as a palpable mass of subareolar breast tissue measuring at least 0.5 cm, 1 cm, or 2 cm by different investigators.4,7
Histopathogenesis
Estrogen and androgen receptors are present in both male and female breasts. Estrogens strongly stimulate the mammary gland, while androgens have a weak inhibitory effect. At birth, male and female breasts are histologically identical, mainly formed by the major lactiferous ducts.8 During childhood, the breast tissue remains quiescent until puberty. At puberty, further differentiation occurs in both sexes. In males, transient proliferation of the ducts and surrounding mesenchymal tissue occurs, presumably due to greater physiological effects of estrogens on breast tissue secondary to a temporary imbalance in the androgen/estrogen ratio.9,10 As puberty advances, circulating levels of androgens rise, leading to involution and atrophy of the ducts.
Etiologies
The etiology of gynecomastia remains unclear. Most cases of gynecomastia are thought to result from an imbalance between estrogens and androgens.1,11 However, in pubertal gynecomastia, the majority of adolescents have normal estrogen levels, although several studies have demonstrated elevated levels in some patients.12,13,14 Pubertal gynecomastia is thought to be a physiological phenomenon, and is most commonly seen in midpuberty with Tanner stage 3–4 pubic hair and testicular volumes of 5 to 10 mL bilaterally.3 In a 3-year longitudinal study of hormonal changes during puberty, study participants with and without gynecomastia were compared.15 No association was found with race, and no significant difference was found in serum estradiol, testosterone, estrogen/testosterone ratio, or dehydroepiandrosterone-sulfate levels.15
Pathological gynecomastia is rare in adolescents and prepubertal-aged boys. It is related to conditions where absolute or relative estrogen excess is present: (1) with exogenous intake, (2) with endogenous production, or (3) with increased peripheral conversion of androgens to estrogens secondary to abundant aromatase activity, androgen deficiency, or androgen insensitivity.3 These are common mechanisms for gynecomastia secondary to medications, adrenal and testicular neoplasms, Klinefelter syndrome, Peutz-Jeghers syndrome, thyrotoxicosis, cirrhosis, primary hypogonadism, congenital adrenal hyperplasia, androgen insensitivity, malnutrition, and aging.3,14 Furthermore, there are conflicting results regarding the presence of a correlation between gynecomastia and obesity.4,15,16 Although the association between these two conditions has not been confirmed, it is known that adipose tissue is an important site of aromatization and estrogen formation, which in theory could support the observation that young men with higher body fat percentage often develop gynecomastia. In the majority of cases of pathological gynecomastia, a specific cause is rarely identified, even after a comprehensive and careful investigation.
Clinical Evaluation
Medical history and physical examination are the most important components of the evaluation of a patient with gynecomastia. A detailed history should focus on time of onset and duration of the gynecomastia, associated symptoms (e.g., mastalgia, bleeding or nipple discharge), presence of systemic disease (especially liver, kidney, adrenal, thyroid, pituitary glands, testes, and prostate), history of recent weight change, presence of risk factors for breast cancer17 (e.g., BRCA2 carriers), and use of medications and recreational drugs (e.g., nonprescription medications, anabolic steroids, dietary supplements, marijuana).
Physical examination should include pubertal development stage, including assessment of voice changes, height increase, testes size, facial and body hair development, penile size and development, and muscle mass increase, and presence of any testicular masses. The breasts should carefully be inspected and palpated for the presence of unusual firmness, asymmetry, nipple discharge, axillary lymphadenopathy, and also to differentiate true gynecomastia from pseudogynecomastia. The normal male breast is relatively flat with a certain degree of fullness around the nipple–areola complex (NAC).18,19 This may vary depending on the degree of chest muscle hypertrophy often seen in athletes and body builders. On average, the nipple is located at 20 cm from the sternal notch in males, and the NAC measures 28 mm.
Following a comprehensive medical history and physical examination findings of age-appropriate physical and sexual development, no further investigation is warranted. Observation and reassurance should be the mainstays of treatment. If gynecomastia is present in prepubertal-aged boys, further investigation should be undertaken to search for endocrinopathy. In male adolescents with gynecomastia, if the physical examination provides signs suggestive of an underlying disorder, diagnostic blood tests to assess serum levels of luteinizing hormone, follicle-stimulating hormone, testosterone, estradiol, prolactin, dehydroepiandrosterone, and human chorionic gonadotropin may be useful.2,3
Classification
Bannayan et al20 have described three histological types of gynecomastia: florid, fibrous, and intermediate. The florid type is characterized by ductal hyperplasia and proliferation, with loose and edematous stroma. The fibrous type contains more stromal fibrosis and fewer ducts. As its name infers, the intermediate type of gynecomastia presents features of the two. In the majority of cases, if the duration of gynecomastia is greater than one year, the fibrous type is more prevalent and irreversible, which may limit success of medical treatments.
Treatment
When an underlying hormonal disorder is identified as the cause of gynecomastia, appropriate treatment should be sufficient to cause regression of breast tissue enlargement. In cases of drug-induced gynecomastia, stopping the offending medication will usually cause regression. Most commonly, the health care provider will be consulted by adolescent boys presenting with pubertal gynecomastia. Pubertal gynecomastia is self-limited in 75 to 90% of adolescents and regresses over 1 to 3 years. Observation and reassurance are widely regarded as the safest and most reasonable treatment. However, because gynecomastia in adolescents occurs at a sensitive time when boys are increasingly aware of their self-image, health care providers may be questioned by the patient and/or his family about the role of pharmacological or surgical therapies.
Pharmacological Treatment
Medical treatment of gynecomastia aims to correct the estrogen-androgen imbalance by three possible pathways: (1) blocking the effects of estrogens on the breast (e.g., clomiphene, tamoxifen, raloxifene), (2) administering androgens (e.g., danazol), and (3) inhibiting estrogen production (e.g., anastrozole, testolactone).3,21
Data on efficacy of pharmacological treatment of gynecomastia in adolescents is mostly limited to small case series and case reports without control groups, which makes conclusions difficult to draw.22,23,24,25,26,27 A randomized double-blind controlled trial by Plourde et al28 found no significant difference in breast volume reduction in male adolescents with pubertal gynecomastia who received anastrozole versus placebo once daily for 6 months. In a small uncontrolled retrospective cohort study assessing the efficacy of tamoxifen and raloxifene in treating persistent pubertal gynecomastia, Lawrence et al29 concluded that raloxifene, and to a lesser extent tamoxifen, may be used successfully to treat this condition. However, an obstacle in studying pharmacologic treatment of pubertal gynecomastia is the difficulty in quantifying treatment effect due to the natural history of spontaneous regression in the majority of adolescents. In light of the available information, current data are inadequate to support pharmacological therapy of persistent pubertal gynecomastia. In such cases, surgery may be considered if no regression is observed after a period of observation of at least one year.
Surgical Treatment
Surgical management of pubertal gynecomastia may be considered in nonobese male adolescents who present persistent breast enlargement after a period of observation of at least 12 months, intractable breast pain or tenderness, and/or significant psychosocial distress. Several classification systems exist, based on clinical features of gynecomastia that may guide the choice of surgical procedure.
In 1973, Simon et al30 identified four grades of gynecomastia:
Grade I: Small enlargement without skin excess
Grade IIa: Moderate enlargement without skin excess
Grade IIb: Moderate enlargement with minor skin excess
Grade III: Marked enlargement with excess skin, mimicking female breast ptosis
Rohrich et al31 have proposed a similar classification of gynecomastia with four grades of severity:
Grade I: Minimal hypertrophy (< 250 g) without ptosis
Grade II: Moderate hypertrophy (250–500 g) without ptosis
Grade III: Severe hypertrophy (> 500 g) with grade I ptosis
Grade IV: Severe hypertrophy with grade II or grade III ptosis
The first report of surgical treatment of gynecomastia dates back to Paulas Aegineta (625–690 AD), a Byzantine Greek physician who performed a reduction mammoplasty through a lunate incision below the breast.32 In 1946, Webster was the first to abandon extra-areolar skin incisions in favor of a semicircular intra-areolar incision.32,33 Current surgical techniques favor standard suction-assisted lipectomy (SAL) and ultrasound-assisted liposuction (UAL)31 over excisional techniques, with the advantage of creating smaller scars. In 1996, Morselli et al34 first described the pull-through technique, which combines SAL in the subcutaneous and subglandular planes, as well as subareolar parenchymal excision. In a 2012 case series of 260 patients aged 10 to 59 years, the authors reported good overall surgical outcomes with low complication rates. Although the authors do not provide the methods for outcome measurement, they report high patient satisfaction with this technique.35 Other surgeons have used the pull-through technique successfully with UAL36 or power-assisted liposuction (PAL).37 In 2010, Petty et al22 reported their experience with UAL and the arthroscopic shaver to resect the subareolar fibrous component.
Because various forms of gynecomastia are encountered in clinical practice, plastic surgeons treating gynecomastia should recognize when excisional and liposuction techniques are needed. The goals of surgical treatment of gynecomastia, regardless of the surgical technique selected, should include a pleasant chest shape with limited scar extension. In general, Simon grade I or IIa/glandular type may be treated with liposuction (SAL, PAL, or UAL), although the Simon grade I or IIa/fibrous type may be better approached with a combination of UAL and surgical excision (Fig. 1). Patients with Simon grade IIb/glandular type may be treated like their Simon grade I or IIa counterparts, and wait 6 to 12 months before considering skin excision (Fig. 2). Simon grade IIb/fibrous type, skin excision may be performed immediately or in a delayed fashion. Lastly, in patients with Simon grade III, any form of liposuction is usually combined with skin resection. In these severe cases, several types of incisions have been reported (e.g., circumareolar incision encompassing the superior or inferior half of the areola, omega incision, concentric circle incision, inframammary incision). In some cases, the nipple-areolar complex may be transposed on a dermoglandular pedicle, or rarely repositioned as a full-thickness skin graft.
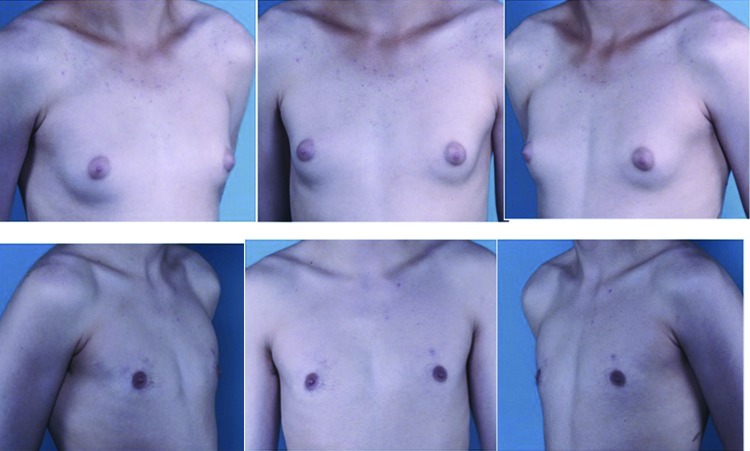
Fig. 1.
Preoperative views of a 16-year-old boy with Simon grade I pubertal gynecomastia with dense breast tissue (upper row). Postoperative result at 15 months (lower row) following ultrasound-assisted liposuction and arthroscopic shaver-assisted removal of the breast tissue.
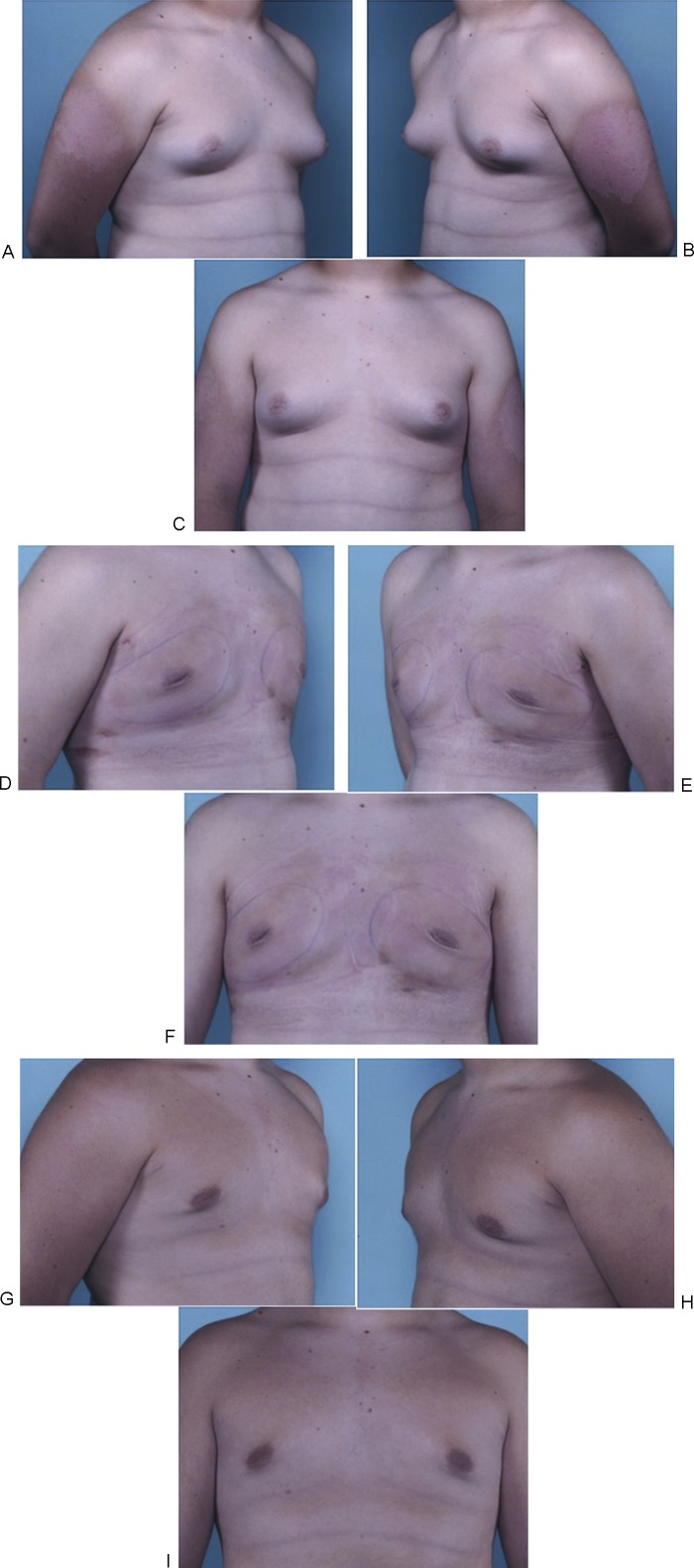
Fig. 2.
(A–C) Preoperative views of a 15-year-old boy with obesity and pubertal gynecomastia. Moderate breast enlargement and minor skin excess (Simon grade IIb) are observed. (D–F) Postoperative views one day after ultrasound-assisted liposuction and arthroscopic shaver assisted removal of the breast tissue without skin excision. (G–I) Postoperative result at 10 months.
The most frequent early complication following surgical management of gynecomastia is hematoma. Seroma, overresection with saucer-type deformity, underresection, unappealing scarring and infections are also observed. Patients and their parents or guardians should be well informed about possible risks, as some complications are managed surgically.
Conclusion
Pubertal gynecomastia is usually self-limited. In evaluating adolescents with gynecomastia, a comprehensive medical history and careful physical examination should be completed. In the majority of cases, observation and reassurance will suffice. Cases of pathological gynecomastia in adolescents and prepubertal gynecomastia are rare. In these two scenarios, further investigation should always be undertaken to rule out an endocrine disorder. When gynecomastia in adolescents persists for more than one year, surgical management may be considered. The surgeon is faced with a range of excisional and liposuction techniques, and the choice of incision and procedure(s) depends on the severity of breast enlargement, the presence of skin excess, and surgeon and patient preference. The method of choice will restore the male chest shape and address skin excess with short, inconspicuous scars. Overall, surgical treatment of gynecomastia appears to provide satisfactory results, although no formal patient-reported outcomes data are available. Pharmacological treatment is not recommended for adolescents suffering from gynecomastia, based on the paucity of data on risks and benefits.
References
- 1.Braunstein G D. Gynecomastia. N Engl J Med. 1993;328(7):490–495. doi: 10.1056/NEJM199302183280708. [DOI] [PubMed] [Google Scholar]
- 2.Bembo S A, Carlson H E. Gynecomastia: its features, and when and how to treat it. Cleve Clin J Med. 2004;71(6):511–517. doi: 10.3949/ccjm.71.6.511. [DOI] [PubMed] [Google Scholar]
- 3.Ma N S, Geffner M E. Gynecomastia in prepubertal and pubertal men. Curr Opin Pediatr. 2008;20(4):465–470. doi: 10.1097/MOP.0b013e328305e415. [DOI] [PubMed] [Google Scholar]
- 4.Kumanov P, Deepinder F, Robeva R, Tomova A, Li J, Agarwal A. Relationship of adolescent gynecomastia with varicocele and somatometric parameters: a cross-sectional study in 6200 healthy boys. J Adolesc Health. 2007;41(2):126–131. doi: 10.1016/j.jadohealth.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Nuttall F Q. Gynecomastia as a physical finding in normal men. J Clin Endocrinol Metab. 1979;48(2):338–340. doi: 10.1210/jcem-48-2-338. [DOI] [PubMed] [Google Scholar]
- 6.Niewoehner C B, Nuttal F Q. Gynecomastia in a hospitalized male population. Am J Med. 1984;77(4):633–638. doi: 10.1016/0002-9343(84)90353-x. [DOI] [PubMed] [Google Scholar]
- 7.Nydick M, Bustos J, Dale J H Jr, Rawson R W. Gynecomastia in adolescent boys. JAMA. 1961;178:449–454. doi: 10.1001/jama.1961.03040440001001. [DOI] [PubMed] [Google Scholar]
- 8.Lemaine V, Simmons P S. The adolescent female: Breast and reproductive embryology and anatomy. Clin Anat. 2013;26(1):22–28. doi: 10.1002/ca.22167. [DOI] [PubMed] [Google Scholar]
- 9.Abaci A, Buyukgebiz A. Gynecomastia: review. Pediatr Endocrinol Rev. 2007;5(1):489–499. [PubMed] [Google Scholar]
- 10.Moore D C, Schlaepfer L V, Paunier L, Sizonenko P C. Hormonal changes during puberty: V. Transient pubertal gynecomastia: abnormal androgen-estrogen ratios. J Clin Endocrinol Metab. 1984;58(3):492–499. doi: 10.1210/jcem-58-3-492. [DOI] [PubMed] [Google Scholar]
- 11.Mathur R, Braunstein G D. Gynecomastia: pathomechanisms and treatment strategies. Horm Res. 1997;48(3):95–102. doi: 10.1159/000185497. [DOI] [PubMed] [Google Scholar]
- 12.Lee P A. The relationship of concentrations of serum hormones to pubertal gynecomastia. J Pediatr. 1975;86(2):212–215. doi: 10.1016/s0022-3476(75)80470-7. [DOI] [PubMed] [Google Scholar]
- 13.LaFranchi S H, Parlow A F, Lippe B M, Coyotupa J, Kaplan S A. Pubertal gynecomastia and transient elevation of serum estradiol level. Am J Dis Child. 1975;129(8):927–931. doi: 10.1001/archpedi.1975.02120450035007. [DOI] [PubMed] [Google Scholar]
- 14.Bidlingmaier F, Knorr D. Plasma testosterone and estrogens in pubertal gynecomastia. Z Kinderheilkd. 1973;115(1):89–94. doi: 10.1007/BF00438995. [DOI] [PubMed] [Google Scholar]
- 15.Biro F M, Lucky A W, Huster G A, Morrison J A. Hormonal studies and physical maturation in adolescent gynecomastia. J Pediatr. 1990;116(3):450–455. doi: 10.1016/s0022-3476(05)82843-4. [DOI] [PubMed] [Google Scholar]
- 16.Sher E S, Migeon C J, Berkovitz G D. Evaluation of boys with marked breast development at puberty. Clin Pediatr (Phila) 1998;37(6):367–371. doi: 10.1177/000992289803700606. [DOI] [PubMed] [Google Scholar]
- 17.Evans D G, Bulman M, Young K. et al. BRCA1/2 mutation analysis in male breast cancer families from North West England. Fam Cancer. 2008;7(2):113–117. doi: 10.1007/s10689-007-9153-9. [DOI] [PubMed] [Google Scholar]
- 18.Beckenstein M S, Windle B H, Stroup R T Jr. Anatomical parameters for nipple position and areolar diameter in males. Ann Plast Surg. 1996;36(1):33–36. doi: 10.1097/00000637-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Murphy T P, Ehrlichman R J, Seckel B R. Nipple placement in simple mastectomy with free nipple grafting for severe gynecomastia. Plast Reconstr Surg. 1994;94(6):818–823. doi: 10.1097/00006534-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Bannayan G A, Hajdu S I. Gynecomastia: clinicopathologic study of 351 cases. Am J Clin Pathol. 1972;57(4):431–437. doi: 10.1093/ajcp/57.4.431. [DOI] [PubMed] [Google Scholar]
- 21.Maidment S L. Question 2. Which medications effectively reduce pubertal gynaecomastia? Arch Dis Child. 2010;95(3):237–239. doi: 10.1136/adc.2009.176768. [DOI] [PubMed] [Google Scholar]
- 22.Petty P M, Solomon M, Buchel E W, Tran N V. Gynecomastia: evolving paradigm of management and comparison of techniques. Plast Reconstr Surg. 2010;125(5):1301–1308. doi: 10.1097/PRS.0b013e3181d62962. [DOI] [PubMed] [Google Scholar]
- 23.Buckle R. Danazol therapy in gynaecomastia; recent experience and indications for therapy. Postgrad Med J. 1979;55 05:71–78. [PubMed] [Google Scholar]
- 24.Buckle R. Studies on the treatment of gynaecomastia with danazol (Danol) J Int Med Res. 1977;5 03:114–123. [PubMed] [Google Scholar]
- 25.Zachmann M, Eiholzer U, Muritano M, Werder E A, Manella B. Treatment of pubertal gynaecomastia with testolactone. Acta Endocrinol Suppl (Copenh) 1986;279:218–226. doi: 10.1530/acta.0.112s218. [DOI] [PubMed] [Google Scholar]
- 26.Derman O, Kanbur N O, Kutluk T. Tamoxifen treatment for pubertal gynecomastia. Int J Adolesc Med Health. 2003;15(4):359–363. doi: 10.1515/ijamh.2003.15.4.359. [DOI] [PubMed] [Google Scholar]
- 27.Derman O, Kanbur N O, Tokur T E. The effect of tamoxifen on sex hormone binding globulin in adolescents with pubertal gynecomastia. J Pediatr Endocrinol Metab. 2004;17(8):1115–1119. doi: 10.1515/jpem.2004.17.8.1115. [DOI] [PubMed] [Google Scholar]
- 28.Plourde P V, Reiter E O, Jou H C. et al. Safety and efficacy of anastrozole for the treatment of pubertal gynecomastia: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89(9):4428–4433. doi: 10.1210/jc.2004-0082. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence S E, Faught K A, Vethamuthu J, Lawson M L. Beneficial effects of raloxifene and tamoxifen in the treatment of pubertal gynecomastia. J Pediatr. 2004;145(1):71–76. doi: 10.1016/j.jpeds.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 30.Simon B E, Hoffman S, Kahn S. Classification and surgical correction of gynecomastia. Plast Reconstr Surg. 1973;51(1):48–52. doi: 10.1097/00006534-197301000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Rohrich R J Ha R Y Kenkel J M Adams W P Jr Classification and management of gynecomastia: defining the role of ultrasound-assisted liposuction Plast Reconstr Surg 20031112909–923., discussion 924–925 [DOI] [PubMed] [Google Scholar]
- 32.Fruhstorfer B H, Malata C M. A systematic approach to the surgical treatment of gynaecomastia. Br J Plast Surg. 2003;56(3):237–246. doi: 10.1016/s0007-1226(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 33.Webster J P. Mastectomy for gynecomastia through a semicircular intra-areolar incision. Ann Surg. 1946;124(3):557–575. [PMC free article] [PubMed] [Google Scholar]
- 34.Morselli P G. “Pull-through”: a new technique for breast reduction in gynecomastia. Plast Reconstr Surg. 1996;97(2):450–454. doi: 10.1097/00006534-199602000-00028. [DOI] [PubMed] [Google Scholar]
- 35.Morselli P G, Morellini A. Breast reshaping in gynecomastia by the “pull-through technique”: considerations after 15 years. Eur J Plast Surg. 2012;35(5):365–371. doi: 10.1007/s00238-011-0595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammond D C Arnold J F Simon A M Capraro P A Combined use of ultrasonic liposuction with the pull-through technique for the treatment of gynecomastia Plast Reconstr Surg 20031123891–895., discussion 896–897 [DOI] [PubMed] [Google Scholar]
- 37.Lista F, Ahmad J. Power-assisted liposuction and the pull-through technique for the treatment of gynecomastia. Plast Reconstr Surg. 2008;121(3):740–747. doi: 10.1097/01.prs.0000299907.04502.2f. [DOI] [PubMed] [Google Scholar]